Scope of DA reaction between 2-furoic acids and maleimides in aqueous solution.
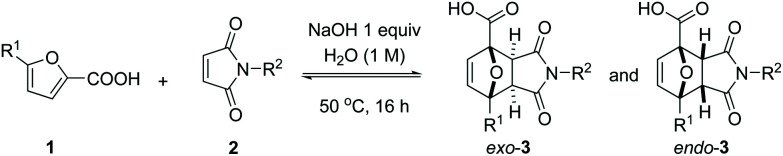
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | 3 | Conv. 1a | exo-3, % | endo-3, % | Isolatedb, % |
| 1 | H | Me | 3a | 98 | 97 | 1 | 77(92)c |
| 2 | H | H | 3b | 95 | 95 | Trace | 68 |
| 3 | H | nPr | 3c | 96 | 93 | 3 | 72 |
| 4d | H | Ph | 3d | 51 | 51 | Trace | 21 |
| 5d | H | Cy | 3e | 18 | 16 | 2 | n.d. |
| 6d,e | H | Cy | 3e | 56 | 53 | 3 | 31 |
| 7 | Me | Me | 3f | 93 | 88 | 5 | 75 |
| 8 | CH2OH | Me | 3g | 91 | 72 | 19 | 51f |
| 9d | CH2OH | Ph | 3h | 28 | 28 | Trace | 11 |
| 10g | CHO | Me | 3i | <10 | ∼5 | Trace | n.d. |
| 11g | COOH | Me | 3j | 20 | 20 | 0 | n.d. |
| 12g,h | COOH | Me | 3j | 56 | 56 | 0 | n.d. |
Conversion determined from the 1H-NMR ratios of product isomers and starting material in the crude mixture.
Isolated yield after acidification.
40 mmol scale.
Poor dissolution of 2.
Methanol was used as cosolvent.
After hydrogenation on Pd/C.
Extensive hydrolysis of 2a to maleic acid.
With 2 equiv. of NaOH.
