Scope of DA reaction between 2-furoic acid derivatives (esters, amides) and maleimides under aqueous conditions.
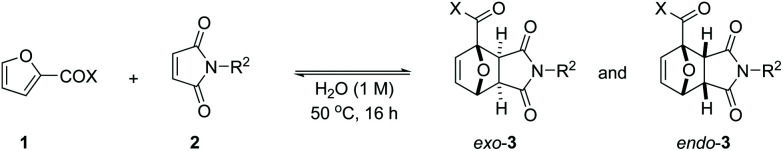
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | X | R2 | 3 | Conv. 1a | exo-3, % | endo-3, % | Isolatedb, % |
| 1 | OMe | Me | 3k | 70 | 65 | 5 | 52(74) |
| 2 | OMe | H | 3l | 67 | 65 | 2 | 43(64)/82c |
| 3 | OMe | Et | 3m | 65 | 61 | 4 | 47(72) |
| 4 | OEt | Me | 3n | 63 | 59 | 4 | 29(46) |
| 5 | OiPr | Me | 3o | 54 | 50 | 4 | 26(49) |
| 6 | OtBu | Me | 3p | 54 | 51 | 3 | 25(46) |
| 7 | NH2 | Me | 3q | 94 | 91 | 3 | 77(83) |
| 8 | NMe2 | Me | 3r | 81 | 77 | 4 | 41(51) |
| 9 | NHOH | Me | 3s | 92 | 76 | 16 | 69(75) |
Conversion determined from the 1H-NMR ratios of product isomers and starting material.
Isolated yield after (chromatographic) workup (in brackets, yield corrected on reacted starting material).
2 M initial concentration.
