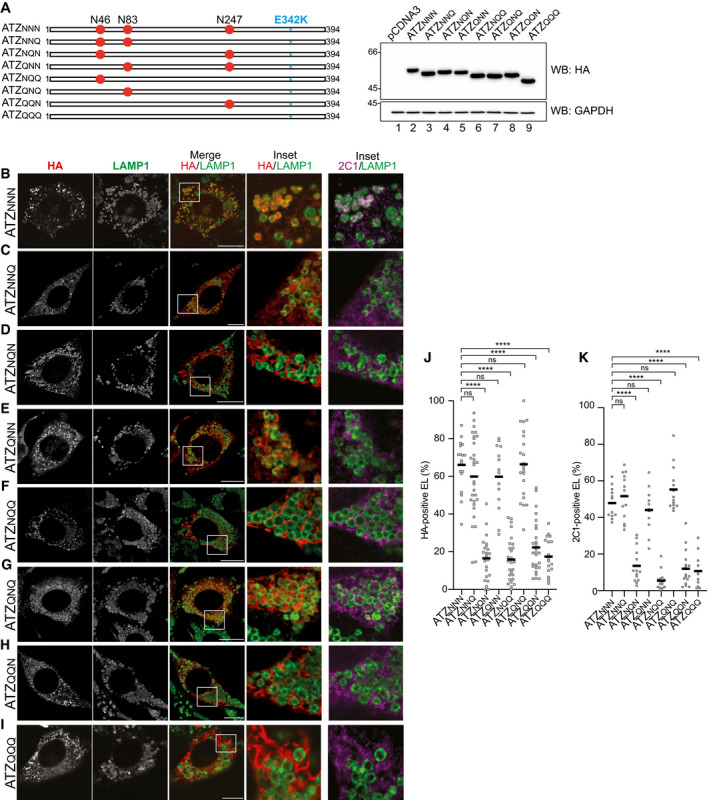
Figure 3. N‐glycosylation at position 83 is required and sufficient for ATZ delivery to endolysosomes.
-
AATZ glycosylation mutants and their electrophoretic mobility. N is the acceptor site asparagine; Q is for the mutation to glutamine that prevents glycosylation. The E342K mutation of ATZNNN is in blue.
-
B–IConfocal laser scanning microscopy analyses (as in Fig 2) in WT MEF treated with BafA1.
-
J, K(J) Quantification of ATZxxx‐positive endolysosomes and (K) of ATZxxx polymers‐positive endolysosomes (mean, n = 18, 30, 24, 14, 27, 22, 27, 18 cells for HA stain and n = 12, 14, 14, 11, 14, 14, 17, 11 cells for 2C1 stain of panels B‐I, respectively). One‐way ANOVA and Dunnett’s multiple comparison test, ns P > 0.05, ****P < 0.0001.
Data information: Scale bars 10 μm.
Source data are available online for this figure.
