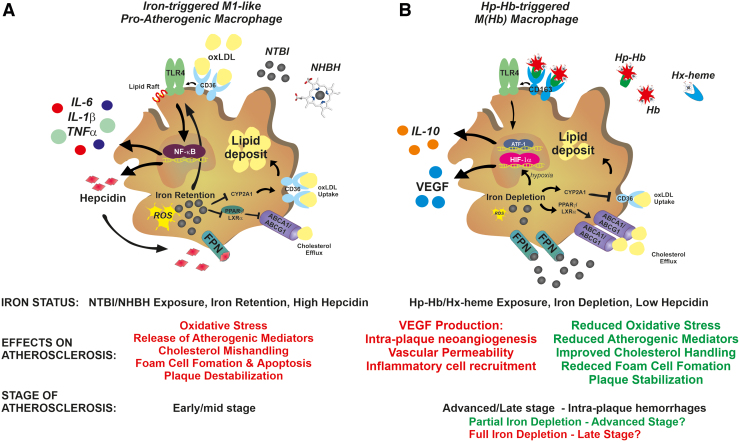
FIG. 5.
Role of macrophage iron content in atherosclerosis. (A) In early/mid-stage atherosclerotic plaques, exposure to NHBH and NTBI directs macrophages toward a proinflammatory phenotypic switching, with potential proatherosclerotic action. Iron and heme stimulate the activation of the TLR/NFkB signaling pathway, which is responsible for macrophage inflammatory activation. In a synergistic manner, oxLDL and iron activate the TLR4 pathway, which in turn triggers the autocrine release of hepcidin. This results in the exacerbation of iron accumulation, ROS production, and inflammatory response through a positive feedback loop. In addition, iron retention accelerates foam cell formation by (i) promoting LDL oxidation and (ii) inducing cholesterol mishandling through increased CD36-mediated cholesterol uptake and decreased ABC transporter ABCA1/ABCG1-mediated reverse cholesterol efflux via interference with the CYP27A1/27HC and PPARγ/LXRα signaling, respectively. Iron and lipids therefore show a synergistic action in accelerating foam cell formation. ROS and TLR4 signaling pathways play major roles in iron-driven macrophage inflammatory activation and foam cell formation, which are prevented by chelators, antioxidants, and TLR4 inhibitors. (B) In advanced hemorrhagic plaques, exposure to the Hp–Hb or Hx–heme complexes directs macrophages toward an iron-recycling phenotype characterized by increased ability to take up Hb via the CD163 receptor and export iron via FPN, which lead to reduced intracellular iron and ROS formation. These macrophages, defined as M(Hb) or Mhem macrophages, show reduced lipid retention, decreased production of inflammatory cytokines, and increased secretion of the anti-inflammatory atheroprotective cytokine IL-10. Reduced intracellular iron and ROS lower inflammatory cytokine production, and improve lipid handling by reducing cholesterol loading via CD36 downregulation and increasing reverse cholesterol efflux via ABC transporter upregulation. While these nonfoam M(Hb) macrophages are in principle atheroprotective, the progressive intracellular iron depletion leads to HIF1α stabilization and VEGF secretion. VEGF exerts proatherosclerotic effects by inducing vascular permeabilization, intraplaque neoangiogenesis, and immune cell recruitment. Whether M(Hb) macrophages play an initial protective effect, which turns into a proatherosclerotic one when severe iron depletion is achieved, remains to be determined. Whereas iron depletion and low hepcidin levels are desirable in early/mid-stage atherosclerosis to activate an atheroprotective phenotypic switching of macrophages, iron balance in late-stage atherosclerosis is preferred to prevent VEGF-related atherosclerotic effects. ABC, ATP-binding cassette; Hb, hemoglobin; HIF1α, hypoxia-inducible factor 1α; Hp, haptoglobin; Hx, hemopexin; ox-LDL, oxidized low-density lipoprotein; VEGF, vascular endothelial growth factor. Color images are available online.