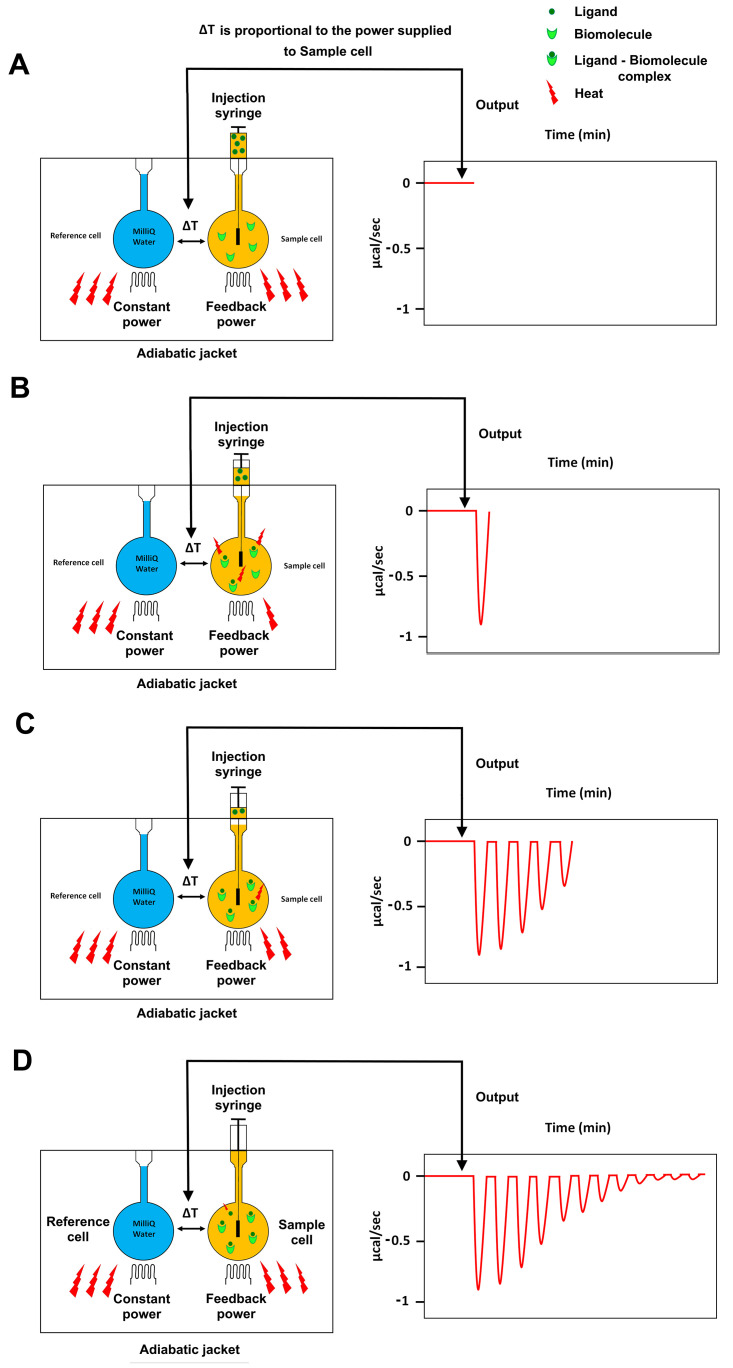
Figure 1. Principle of Isothermal Titration Calorimetry (ITC).
A. Cartoon representation of an isothermal titration calorimetry instrument composed of: a reference cell filled with MilliQ water; a sample cell containing a biomolecule; and an automated injection syringe containing the other binding molecule (ligand) used to titrate the ligand into the sample cell. The sample and reference cells are surrounded by an adiabatic jacket. The system is able to detect temperature differences between the reference and sample cells and to maintain an absence of temperature difference between them (ΔT = 0) by supplying power to both the reference and the sample cell via two heaters. The output of the instrument is the power (μcal/sec) required to maintain ΔT = 0 between the reference and sample cells. B-D. The temperature difference between the reference and the sample cell, induced by the ligand–biomolecule binding, is converted into the power needed to bring the two cells back to the same temperature during the binding reaction. As the titration proceeds, the biomolecule in the sample cell becomes saturated with the ligand, so that less interactions occur and consequently the heat change decreases (C) until the biomolecule is fully saturated and the instrument detects only heat change due to the dilution of the ligand (D).