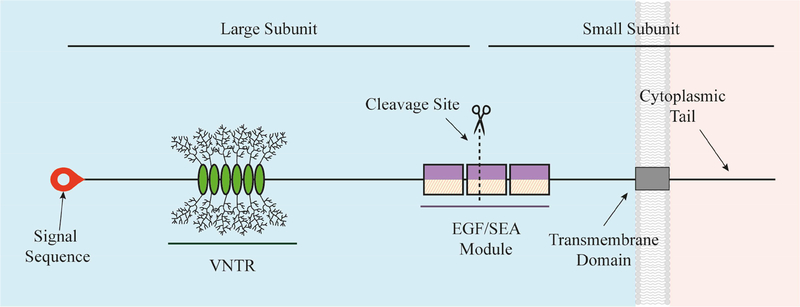
Figure 1.
Molecular Prototype of a Membrane-Associated Mucin.
The graphic depicts a prototypical MAM, the structure of which is like a classic, single-pass transmembrane immune receptor. A signal peptide is found at the N-terminus of the precursor polypeptide chain to enable its membrane insertion; it may be retained in the mature protein [14]. The mature protein is composed of two subunits that self-associate, arising from intracellular cleavage. The large subunit is entirely extracellular and contains the VNTR module with its tandem repeat that are O-glycosylated. The small subunit consists of a short extracellular region, a single-pass transmembrane domain, and a cytoplasmic tail (CT). The large subunit of the MAM, together with the extracellular portion of the small subunit, comprise the extracellular domain (ED). This domain also contains conserved modules such as the Sperm protein, Enterokinase and Agrin module (SEA) and EGF-like modules.