Abstract
Purpose:
To examine whether “activated” dendritic cells (aDCs) could serve as a biomarker of systemic immune disorders in individuals with dry eye (DE) symptoms. Secondarily, to examine the impact of a topical anti-inflammatory agent on aDC number.
Methods:
Retrospective analysis was conducted to identify individuals with DE symptoms who had in-vivo confocal microscopy (IVCM) imaging between October 2018 and July 2020 at the Miami Veterans Hospital. aDCs were manually quantified based on morphology. Receiver operating curve (ROC) analysis examined relationships between aDC number and systemic immune disease status. Individuals were then grouped by aDC number (≥2 versus <2) and demographics and DE parameters were examined. Paired t-test was performed to evaluated aDC number pre- vs post-initiation of an anti-inflammatory agent.
Results:
128 individuals were included. Their mean age was 57.1±15.0 years; 71.1% were male, 53.1% self-identified as White and 24.2% as Hispanic. The mean number of aDCs in the central cornea was 1.28±2.16 cells/image. The presence of ≥2 aDCs had a sensitivity of 60% and specificity of 77% for the diagnosis of a systemic immune disorder. Individuals with ≥2 aDCs were more likely to self-identify as Black, have Secondary Sjögren’s, and have higher nerve fiber area and fractal dimension. In 12 individuals, aDC number decreased from 2.69±2.36 to 0.58±0.73 cells/image after initiation of an anti-inflammatory agent, p=0.01.
Conclusions:
The presence of ≥2 aDCs in the central cornea suggests a systemic immune disorder in individuals with DE symptoms. Topical anti-inflammatory therapy can reduce the number of aDCs in the central cornea.
INTRODUCTION:
Dry Eye (DE) is a common disease that affects over 16 million people in the United States (US).1 DE can manifest with symptoms of pain, described as dryness, burning, and foreign body sensation; and/or visual disturbances, described as poor or fluctuating vision. Signs of DE are likewise varied and include decreased tear production, increased tear instability, epithet lial irregularities, and ocular surface inflammation. 2,3 Adding to the complexity, many studies have found a disconnect between symptoms and signs of disease.4 Overall, DE symptoms interfere with activities of daily living (such as reading and computer work), have a negative effect on mental health, and decrease overall quality of life.5, 6 The disease is more prevalent among women and its incidence increases with age.4 Many factors can influence DE symptoms and signs including co-morbid systemic immune conditions, meibomian gland dysfunction (MGD), the use of topical and oral medications, and surgery.4 A challenge in DE is to identify the specific abnormalities that contribute to symptoms and signs of disease in an individual patient.
One potential biomarker in DE is the presence of dendritic cells (DCs) in the cornea. DCs are antigen presenting cells (APCs) that play an important role in the innate and adaptive immune responses7. In a normal cornea, DCs are mostly found peripherally, within the anterior stroma and epithelium.8,9 In response to inflammation, DCs undergo several changes that have been termed “activation”. These include changes in morphology (such as an increase in overall size, and increased number and length of dendrites10), location (migration into the central cornea10), and expression of surface markers (MHC-II, CD83, CD86 and CCR7).11 Studies have demonstrated that “activated” Dendritic Cells (aDCs) migrate to the cervical lymph nodes and promote the differentiation of naïve T-cells, which then find their way to the cornea. 12,13 This process has been noted to occur in various animal models of DE.14, 15 In a desiccating stress model, expression of maturation markers on DCs was accompanied by an increase of CD4+ T- cells within the cervical lymph nodes and cornea.11 Similar findings were demonstrated in a mice model of Sjögren’s (TSP-1 null mice) where DE develops spontaneously.16 In addition, the important role of DCs as APCs in DE was demonstrated by a higher percent of DCs in the cervical lymph node expressing an antigen placed on the cornea in TSP-1 null, compared to control mice.16
The morphology and location of DCs can also be evaluated in humans with in-vivo confocal microscopy (IVCM).17 Specifically, metrics that can be captured using IVCM include number of DCs in the central cornea (usually represented as cells/mm2 or cells/image), size of DCs, and number and length of dendrites. Overall, individuals with Sjögren’s-associated DE have been found to have a higher number of DCs (71.65±72.54 to 169±48 cells/mm2) compared to individuals with non-Sjögren’s DE (40.33±31.63 to 89.8±10.8 cells/mm2)18–23, MGD (49.04±55.68 to 82±38 cells/mm2)24–26, and controls (21.46±21.74 to 53±34 cells/mm2).19–23, 25, 27–29 Beyond Sjögren’s, individuals with other autoimmune diseases have been found to have higher numbers of DCs in the central cornea, including those with rheumatoid arthritis (25.9±26.6 to 68.2±72.3 cells/mm2),21, 28 systemic lupus erythematosus (43.08±48.67 cells/mm2),30 and Behcet’s (19.6 [6.3–46.3] cells/mm2),31 compared to healthy controls (10.1 to 23.85 ± 33.81 cells/mm2).28, 30, 31
Interestingly, the morphological characteristics of “activation” (size, number, and length of dendrites) has also been associated with immunohistochemistry (IHC) markers of “activation” (HLA-DR) in humans. In a study of 41 individuals that had an IVCM scan prior to penetrating keratoplasty due to different etiologies (herpetic keratitis, keratoconus, or prior graft rejection), the morphology of DCs on IVCM was compared to markers noted on IHC. “Immature” or “inactive” DCs (DC-SIGN+, HLA-DR-) had “bright cell bodies with shorter plumb dendrites” on IVCM and were predominant in individuals with keratoconus; while “mature” or “active” DCs (DC-SIGN+, HLA-DR+) had “longer interdigitating dendrites” and were more predominant in individuals with inflamed corneas (history of herpetic keratitis or graft rejection)32.
Based on the data above, the goal of this study was to examine whether DC number could be used clinically to identify individuals with DE in the setting of a co-morbid systemic immune condition. As there are no built-in software packages to quantify DC number, clinicians usually rely on manual counts. Given that aDC numbers are lower than total DC numbers (and therefore quicker to quantify manually) and that the two metrics are correlated19, 21, our current study examined whether aDC number could be used as a biomarker for the presence of a systemic immune disorder in individuals with DE symptoms. Secondarily, we evaluated whether aDC numbers decreased after initiation of a topical anti-inflammatory agent, irrespective of the presence or absence of a co-morbid autoimmune disease.
METHODS:
2.1. Study population
Retrospective chart review was conducted to identify individuals seen at the Miami Veterans Administration Medical Center with DE symptoms (Dry Eye Questionnaire (DEQ-5) ≥6) and an IVCM scan between October 2018 and July 2020. Individuals were excluded if they had ocular co-morbidities that could confound the ability to detect aDCs on IVCM, such as corneal scarring or a history of corneal infection. 33,34 Data regarding demographics and pertinent medical information including ocular and systemic comorbidities, surgical history, and current medications were obtained. A total of 12 individuals with DE symptoms were started on topical 0.05% cyclosporine therapy twice a day combined with fluorometholone 0.1% for the first month and had a repeat scan ≥3 months after commencing therapy.
2.2. Clinical assessment
All individuals seen in the dry eye clinic underwent a standard evaluation which included filling out validated questionnaires for dry eye symptoms: Dry Eye Questionnaire-5 (DEQ-5; 0–22)35, Ocular Surface Disease Index (OSDI; 0–100)36, and 4 select questions from the NPSI-Eye37 regarding intensity of burning pain, evoked pain to wind, light and changes in temperature (scale 0–10 for each question)38. The ocular surface examination included, in the order performed (1) matrix metalloproteinase 9 test (MMP-9; InflammaDry; Quidel, San Diego, CA; 0–3); (2) eyelid evaluation for anterior blepharitis (scale 0–3), vascularity (scale 0–3), Meibomian gland inspissation (scale 0–3); (3) fluorescein placed and conjunctivochalasis evaluated (temporal, middle and nasal; scale 0–2); (4) tear break-up time (TBUT), 3 values measured in each eye and averaged in seconds; (5) corneal epithelial cell disruption graded to the NEI scale (0–15)39; (5) palpebral conjunctiva morphology graded (scale 0–2); (6) ocular pain assessment via numerical rating scale (NRS, 0–10)40 before and after topical anesthesia; (7) basal tear secretion (Schirmer’s test with anesthesia measured in mm of wetting at 5 minutes); and (8) meibum quality (scale 0–4). A repeated evaluation including DEQ-5, OSDI and MMP-9 measurements was conducted for individuals who received a second scan after commencing topical anti-inflammatory therapy.
2.3. In vivo Confocal microscopy
Laser scanning in vivo microscopic was conducted using the Rostock Cornea Module of the Heidelberg Retina Tomograph III (Heidelberg Engineering, Heidelberg, Germany) to capture images of the central cornea of all patients as previously described.33 This confocal microscope utilizes a 670 nm wavelength Helium-Neon diode laser as the illumination source and a 63x objective immersion lens. Each patient received a drop of 0.5% proparacaine hydrochloride as topical anesthetic before the examination. Patients were properly positioned and instructed to fixate on a target light. An appropriate amount of 0.3% Hypromellose gel (Systane Lubricant Eye Gel, Alcon, Fort Worth, TX) was applied to the lens tip and a disposable sterile plastic cap (Tomo-Cap, Heidelberg Engineering, Heidelberg, Germany) for lubrication and to improve optical coupling. The lens was moved toward the eye until the gel contacted the central cornea. Maximum image acquisition time was set at 5 minutes per eye. A total of 5 sequence scans of non-overlapping areas of the central cornea with a target depth of 30–60 μm were recorded at a rate of 30 frames per second, yielding up to 100 images per scan for each patient. Each image represented a coronal section measuring 400 μm by 400 μm, with 1–2 μm lateral resolution and 4 μm axial resolution.
Representative sub-basal nerve plexus images were selected by reviewers masked to the clinical findings using the following selection criteria: 1) best focused complete image of a single plane located between Bowman’s layer and the basal epithelial membrane; 2) absence of significant artifacts (such as motion or folds); 3) adequate contrast allowing appropriate nerve detection. A total of three non-overlapping images was obtained per patient and the averages of the obtained values were used for analyses.
2.4. Quantitative image analysis
Quantitative image analysis was performed using the automated ACCMetrics Corneal Nerve Fiber Analyzer software v.2 (University of Manchester, Manchester, United Kingdom)41 which has been previously validated.42 The parameters analyzed included: Corneal Nerve Fiber Density defined as the total number of major nerve fibers per squared millimeter (fibers/mm2); Corneal Nerve Fiber Length defined as the total length of all nerve fibers per squared millimeter (mm/mm2); Corneal Nerve Branch Density defined as the number of branches originating from major nerve trunks per squared millimeter (branches/mm2); Corneal Total Branch Density defined as the total number of branch points per squared millimeter (branches/mm2); Corneal Nerve Fiber Area defined as the total nerve fiber area in squared millimeters per squared millimeter (mm2/mm2); Corneal Nerve Fiber Width defined as the average nerve fiber width per squared millimeter (mm/mm2); and Corneal Nerve Fractal Dimension that measures the structural complexity of corneal nerves.43 The nerve tracings of all images analyzed automatically by the software were reviewed and corroborated by the investigators.
2.5. Qualitative image analysis
Qualitative analysis was conducted using the same images to evaluate DCs based on their morphological appearance. Up to three images per individual were counted by reviewers masked to the clinical findings and DCs and aDCs were quantified. DCs were identified as hyperreflective cells with or without prominent processes emanating from them. DCs were categorized as “activated” if they had a slender body with at least 3 processes emanating from the cell trunk that were of the same size or longer than the cell body itself. 32, 44 Representative images demonstrating the selection parameters of aDCs are shown in Figure 1. Total DCs and aDCs were reported as the number per image (cells/image). For comparison to prior studies, this number was adjusted by a factor of 6.25 to report as cells/mm2 given that each image measured 400μm by 400 μm. Prior to commencing the study, we first examined inter-rater reliability using the intra-class correlation coefficient (ICC). Two masked readers evaluated 20 images with an ICC of 0.981 (p<0.001) for DC number and 0.948 (p<0.001) for aDC number.
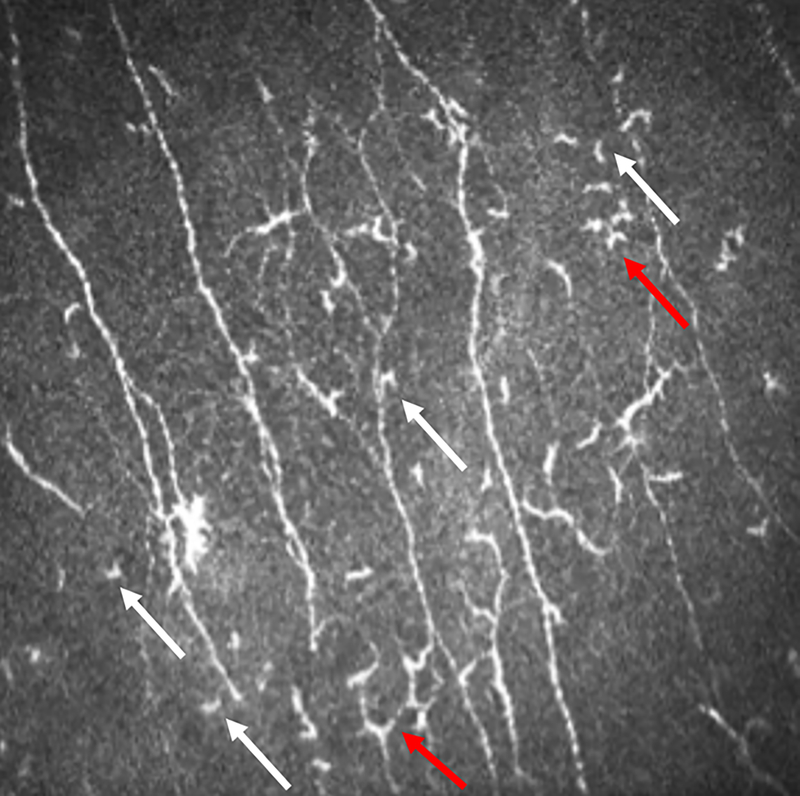
Figure 1.
Morphological evaluation of dendritic cells.
“Activated” dendritic cells are denoted by red arrows which were differentiated from non-activated dendritic cells (white arrows) by the number and size of their arms.
2.6. Statistical Analysis
Statistical analyses were performed using SPSS statistical package version 26.0 (IBM Corp, Armonk, NY). In this study, confocal data from the right eye of each individual were included. Receiver operator curve (ROC) analysis was conducted to examine the aDC cut-off that best discriminated between individuals with vs without a known systemic immune disease. Area under the curve, standard error (SE), 95% Confidence intervals (CI), and sensitivity and specificity were reported. Based on the ROC analysis, individuals were grouped based on the presence or absence of ≥2 aDC in the central cornea. Independent two-tailed Student t-test, Chi square and Fischer’s exact tests were used, as appropriate, to compare variables of interest between the 2 groups. A forward stepwise binomial logistic regression was then conducted to evaluate the contribution of possible confounders to a diagnosis of autoimmune disease. For this analysis, aDC number was dichotomized (≥2aDCs vs <2aDC). Paired two-tailed Student t-test was utilized to compare variables before and after the initiation of a topical anti-inflammatory agent in a subset of individuals (n=12). P-value less than 0.05 was considered statistically significant.
2.7. Ethical statement
This study was approved by the Institutional Review Board (IRB) at the Miami Veterans Administration Medical Center and was conducted in accordance with the principles of the Declaration of Helsinki and the United States Health Insurance Portability and Accountability Act.
RESULTS
Study population:
A total of 128 individuals were included in this study. Demographic characteristics of the study population are presented in Table 1. Among the study population, the mean age was 57.1±15.0; 91 (71.1%) were male; 68 (53.1%) self-identified as White and 31 (24.2%) as Hispanic; 26 (20.3%) were current tobacco smokers.
Table 1:
Demographic information of the study population
| Number | 128 |
|---|---|
| Age (years), mean ±SD [range] | 57.1±15.0 [25–90] |
| Sex, male, n (%) | 91 (71.1%) |
| Ethnicity, Hispanic, n (%) | 31 (24.2%) |
| Race, n (%) | |
| White | 68 (53.1%) |
| Black | 43 (33.6%) |
| Asian | 3 (2.3%) |
| American Indian/Alaska native | 1 (0.8%) |
| Native Hawaiian/ Pacific Islander | 4 (3.1%) |
| Current Smoker, n (%) | 26 (20.3%) |
SD=standard deviation; n=number in group
Numbers of DCs and aDCs in the central cornea:
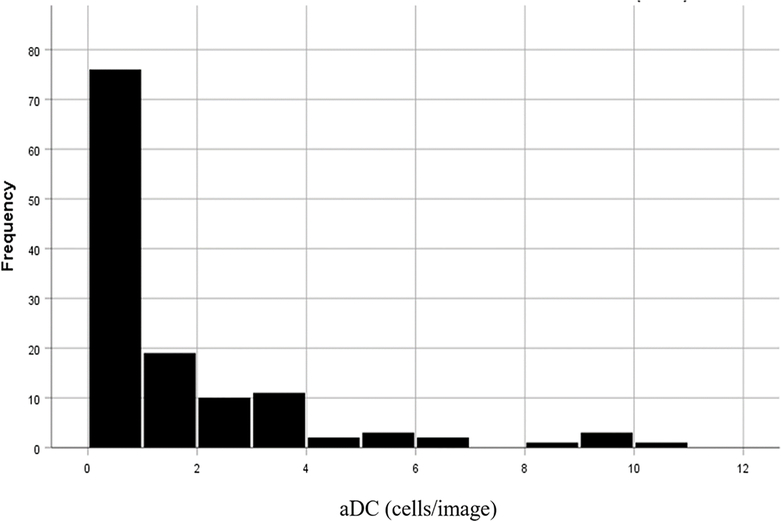
The mean number of DCs in the central cornea was 5.67±7.5 cells/image (35.4±46.9 cells/ mm2), range [0–48 cells/image; 0–300 cells/mm2]. The mean number of aDCs in the central cornea was 1.28±2.16 cells/image (8.0±13.5 cells/mm2), range [0–10.5 cells/image; 0–65.6 cells/mm2] (Figure 2).
Figure 2:
Distribution of the number of activated dendritic cells (aDCs) in the central cornea of individuals with DE symptoms.
Receiver operator curve analysis:
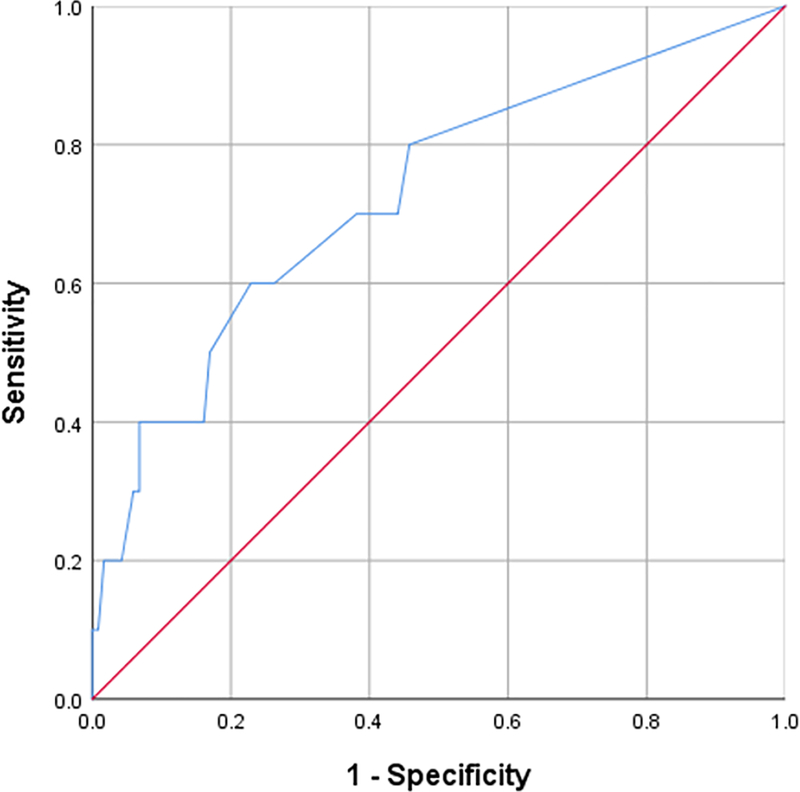
13 of 128 individuals were diagnosed with a systemic immune disease including Sjögren’s, Graft versus host disease (GVHD), autoimmune vasculitides (temporal arteritis, granulomatosis with polyangitis, Behcet’s syndrome), sarcoidosis, rheumatoid or psoriatic arthritis, systemic lupus erythematosus, and/or psoriasis. ROC analysis was conducted to evaluate the sensitivity and specificity of aDC for grouping individuals into systemic immune disease categories (absence vs present) (Figure 3). The area under the ROC curve was 0.73 (SE 0.09; 95% CI 0.55–0.90; p-0.02). The ROC curve analysis suggested that the most useful cutoff was ≥2 aDCs per image, with a sensitivity of 60% and a specificity of 77%.
Figure 3:
ROC curve of the number of activated dendritic cells (aDCs) in the central cornea as an indicator of systemic immune disease.
Demographics and co-morbidities by aDCs number:
Based on the ROC analysis, we then split individuals into two groups based on the presence or absence of aDCs≥2 in the central cornea. Demographics, systemic and ocular comorbidities, and medications split by aDC≥2 are presented in Table 2. Black individuals were more likely than White individuals to have ≥2aDCs in their central cornea (51.5% vs 27.7%, p=0.02). Age, sex, and ethnicity were similarly distributed between the two groups. Similar to the ROC analysis, individuals with vs without systemic immune diseases were more likely to have ≥2aDCs in their central cornea (23.3% vs 7.5%, p=0.04), with this relationship mostly driven by secondary Sjögren’s (15.2% vs 3.2%, p=0.03). Individuals with ≥2aDCs in the central cornea were more likely to be treated with autologous serum tears (30.3% vs 9.5% p<0.01) and oral immunosuppressants (15.2% vs 2.1% p<0.01).
Table 2:
Descriptive statistics for individuals with dry eye (DE) symptoms grouped by the presence or absence of ≥2 activated dendritic cells (aDCs) in the central cornea.
| Parameter | <2aDCs (n=95) | ≥2 aDCs (n=33) | P-Value |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ±SD | 57.5±15.5 | 56.1±13.5 | 0.62 |
| Male sex, n (%) | 68 (71.6%) | 23 (69.7%) | 0.83 |
| Hispanic, n (%) | 17 (23.4%) | 9 (27.3%) | 0.64 |
| Race, White, n (%) | 56 (59.6%) | 12 (36.4%) | 0.02 |
| Black, n (%) | 26 (27.7%) | 17 (51.5%) | 0.02 |
| Medical history, n (%) | |||
| Current Smoker | 22 (23.2%) | 4 (12.1%) | 0.21 |
| Major Depressive Disorder | 37 (38.9%) | 9 (27.3%) | 0.29 |
| Post-Traumatic Stress Disorder | 16 (16.8%) | 11 (33.3%) | 0.08 |
| Hypertension | 49 (51.6%) | 19 (57.6%) | 0.69 |
| Diabetes Mellitus | 20 (21.1 %) | 9 (27.3%) | 0.48 |
| Migraine | 36 (37.9%) | 14 (42.4%) | 0.68 |
| Fibromyalgia | 10 (10.5%) | 6 (18.2%) | 0.36 |
| Traumatic Brain Injury | 4 (4.2%) | 0 (0%) | 0.57 |
| Systemic immune Disease* | 6 (7.5%) | 7 (23.3%) | 0.04 |
| Primary Sjögren’s | 2 (2.1%) | 1 (3.0%) | >0.99 |
| Secondary Sjögren’s | 3 (3.2%) | 5 (15.2%) | 0.03 |
| Positive early Sjögren’s markers | 18 (18.9%) | 9 (27.3%) | 0.33 |
| Ocular History, right eye only, n (%) | |||
| Glaucoma | 9 (9.5%) | 3 (9.1%) | >0.99 |
| Glaucoma Surgery** | 2 (2.1%) | 1 (3.0%) | >0.99 |
| Cataract Surgery | 15 (15.8%) | 7 (21.2%) | 0.59 |
| Refractive surgery | 15 (15.8%) | 1 (3.0%) | 0.07 |
| Systemic Medications, n (%) | |||
| Anti-hypertensive | 50 (47.4%) | 19 (57.6%) | 0.69 |
| Glucose lowering medication | 15 (15.8%) | 7 (21.2%) | 0.59 |
| Inhaled corticosteroids | 15 (15.8%) | 2 (6.1%) | 0.24 |
| Oral corticosteroids | 5 (5.3%) | 2 (6.1%) | 1.00 |
| Immunosuppressive agents (tacrolimus, methotrexate, cyclosporine, azathioprine, mycophenolate) | 2 (2.1%) | 5 (15.2%) | 0.01 |
| NSAID | 39 (41.1%) | 14 (42.4%) | 0.23 |
| Acetaminophen | 13 (13.7%) | 7 (21.2%) | 0.40 |
| α2γ ligand (gabapentin or pregabalin) | 32 (33.7%) | 16 (48.5%) | 0.15 |
| Anti-migraine (triptan) | 10 (10.5%) | 6 (18.2%) | 0.36 |
| Anti-depressant (SSRI, SNRI, mirtazapine, TCA) | 41 (43.2%) | 15 (45.5%) | 0.84 |
| Doxycycline | 10 (10.5%) | 2 (6.1%) | 0.73 |
| Topical Medications, n (%) | |||
| Artificial Tears | 72 (75.8%) | 25 (75.8%) | >0.99 |
| Antihistamine | 12 (12.5%) | 2 (6.1%) | 0.52 |
| Anti-inflammatory (cyclosporine, lifitegrast, corticosteroid, NSAID) | 48 (50.5%) | 17 (51.5%) | >0.99 |
| Autologous Serum Tears (AST) | 9 (9.5%) | 10 (30.3%) | <0.01 |
| IOP lowering | 4 (4.2%) | 3 (9.1%) | 0.37 |
SD=standard deviation; n=number in group, GVHD=Graft versus host disease, NSAID=non-steroidal anti-inflammatory drug; SSRI=Selective serotonin reuptake inhibitor; SNRI=Serotonin-norepinephrine reuptake inhibitor; TCA=tricyclic antidepressants; IOP=intraocular pressure
Includes autoimmune vasculitides (temporal arteritis, granulomatosis with polyangitis, Behcet’s syndrome), sarcoidosis, rheumatoid or psoriatic arthritis, systemic lupus erythematosus, psoriasis, Sjögren’s and GVHD.
Excludes peripheral iridotomy.
A binary logistic regression with forward stepwise analysis was performed to evaluate the contributions of demographics (age, sex, race and ethnicity), smoking status and comorbidities on the diagnosis of a systemic autoimmune disease. Female sex (OR: 7.62; 95% CI 2.07–28.09; p=0.002) and the presence of ≥2aDC (OR: 4.52; 95% CI 1.27– 16.04; p=0.02) remained significant predictors for the diagnosis of concomitant autoimmune disease and these variables accounted for approximately 24% of the variance in the model (R=0.49, p<0.001).
DE profiles by aDC number:
DE symptoms, signs and IVCM parameters grouped by the presence or absence of ≥2 aDCs are presented in Table 3. DE symptoms and signs were similar between the groups. The exception was a lower frequency of conjunctivochalasis in individuals with ≥2 aDCs. Not surprisingly, individuals with ≥2 aDCs also had a higher number of overall DC compared to those with <2aDCs. Furthermore, nerve fiber area and fractal dimension were also higher in the ≥2 aDCs vs < 2 aDCs group (0.006±0.002 vs 0.007±0.003 μm2/mm2, p<0.01 and 1.45±0.06 vs 1.47±0.04, p=0.05, respectively).
Table 3:
Symptoms, signs and in-vivo confocal microscopy (IVCM) parameters of individuals with dry eye (DE) symptoms grouped by the presence or absence of ≥2 activated dendritic cells (aDCs) in the central cornea.
| Parameter | <2aDCs (n=95) | ≥2 aDCs (n=33) | P-Value |
|---|---|---|---|
| Dry eye specific questionnaires, mean (SD) | |||
| Total DEQ-5 Score | 15.1 (3.6) | 16.1 (4.2) | 0.19 |
| Total OSDI Score | 49.2 (25.3) | 57.5 (21.0) | 0.10 |
| Ocular pain specific questionnaires | |||
| Intensity of ocular pain, averaged over past | 4.6 (2.9) | 4.7 (2.7) | >0.99 |
| week, mean (SD) | |||
| NPSI-E sub-score*, mean (SD) | 18.5 (11.6) | 18.2 (10.0) | 0.88 |
| Intensity of burning, mean (SD) | 4.5(3.3) | 4.8(3.2) | 0.71 |
| Burning ≥ 8, n (%) | 24 (25.3%) | 8 (25.0%) | 0.99 |
| Wind sensitivity, mean (SD) | 4.6 (3.3) | 0.7 (2.6) | 0.78 |
| Wind sensitivity ≥ 8, n (%) | 20 (24.2%) | 4 (12.5%) | 0.21 |
| Light sensitivity, mean (SD) | 5.1(3.4) | 5.4(3.3) | 0.62 |
| Light sensitivity ≥ 8, n (%) | 31 (33.0%) | 11 (34.4%) | 0.99 |
| Temperature sensitivity, mean (SD) | 4.4 (3.2) | 3.9 (2.9) | 0.41 |
| Temperature sensitivity ≥ 8, n (%) | 18 (19.1%) | 3 (9.4%) | 0.28 |
| Persistent Pain After Anesthesia**, n (%) | 53 (55.8%) | 21 (63.6%) | 0.54 |
| Ocular surface finding | |||
| MMP-9, mean (SD) | 1.0 (0.9) | 1.1 (1.0) | 0.67 |
| Anterior blepharitis, mean (SD) | 0.6(0.8) | 0.5 (0.7) | 0.79 |
| Eyelid vascularity, mean (SD) | 1.0 (1.1) | 0.7 (1.0) | 0.10 |
| Meibomian gland inspissation, mean (SD) | 0.8 (0.7) | 0.8 (0.7) | 0.89 |
| Temporal conjunctivochalasis, mean (SD) | 0.6 (0.6) | 0.4 (0.5) | 0.19 |
| Middle conjunctivochalasis, mean (SD) | 0.1 (0.3) | 0 (0) | <0.01 |
| Nasal conjunctivochalasis, mean (SD) | 0.3 (0.5) | 0.1 (0.3) | <0.01 |
| Tear break up time (s), mean (SD) | 6.0 (4.0) | 5.9 (4.0) | 0.89 |
| Corneal staining, mean (SD) | 2.1 (2.7) | 3.1 (4.2) | 0.20 |
| Schirmer wetting length (mm/5min) | 9.6 (7.4) | 8.1 (5.7) | 0.30 |
| Papillae, mean (SD) | 0.5 (0.5) | 0.4 (0.5) | 0.82 |
| Fibrosis, n (%) | 5 (6.0%) | 0 (0%) | 0.32 |
| Meibum quality, mean (SD) | 1.1 (1.1) | 1.1 (1.1) | >0.99 |
| Central cornea IVCM Parameters, mean (SD) | |||
| Nerve fiber density (fibers/mm2) | 19.8 (8.8) | 18.3 (9.4) | 0.42 |
| Nerve fiber length (mm/mm2) | 12.0 (3.8) | 13.0 (4.0) | 0.18 |
| Nerve branch density (branches/mm2) | 24.7 (17.4) | 24.5 (16.3) | 0.96 |
| Total branch density (branches/mm2) | 38.3 (26.1) | 45.8 (32.1) | 0.24 |
| Nerve fiber area (mm2/mm2) | 0.006 (0.002) | 0.007 (0.004) | <0.01 |
| Nerve fiber width (mm/mm2) | 0.021 (0.002) | 0.022 (0.002) | 0.12 |
| Nerve fractal dimension | 1.45 (0.06) | 1.47 (0.04) | 0.05 |
| Dendritic cells (cells/mm2) | 20 (25.6) | 79.4 (63.1) | <0.01 |
| Activated dendritic cells (cells/mm2) | 1.7 (2.8) | 26.3 (15.0) | <0.01 |
SD = Standard Deviation; DEQ-5 = Dry Eye Questionnaire 5; OSDI = Ocular Surface Disease; aDC=activated Dendritic Cells; MMP-9= matrix metalloproteinase 9 test
NPSI-E Sub-Score= Total score of four select questions from the Neuropathic Pain Symptom Inventory modified for the Eye referring to the intensity of burning, wind sensitivity, light sensitivity, temperature sensitivity; NRS=numerical rating scale
NRS Pre-Anesthesia > 0 and NRS Post-Anesthesia > 0
Change in aDCs with treatment:
A subset of the population with DE symptoms (n=12), independent of the presence or absence of a systemic immune disease, were started on topical anti-inflammatory therapy and had a repeat scan ≥3 months after commencing therapy. The average time interval between initiation of therapy and repeat scan was 5.1±1.5 months. The aDCs number in the central cornea decreased by 78% from 2.69±2.36 cells/image (16.81±14.75 cells/mm2) to 0.58±0.73 cells/image (3.63±4.56 cells/mm2), p=0.01. There were no significant changes in symptom scores over time (via the DEQ-5 and OSDI) in these individuals: however, the MMP-9 score decreased from 1.40±0.70 to 0.90±0.57, p=0.05.
DISCUSSION
In this study, we present the clinical utility of using aDC number in the central cornea as a biomarker of a co-morbid systemic immune disease in individuals with DE symptoms. This is needed, as an important consideration in DE is to determine whether a systemic immune disease contributes to symptoms and/or signs. Prior studies have demonstrated that 18–35% of individuals with an autoimmune disease, such as Sjögren’s, rheumatoid arthritis, systemic lupus erythematosus or thyroid disorders, have DE symptoms.45–49 Many individuals already carry a systemic diagnosis when presenting to the eye care provider, but in some cases, DE is diagnosed prior to a systemic autoimmune disease. This most commonly occurs with primary Sjögren’s,50 but can happen with other diseases, such as thyroid abnormalities.51,52 In Sjögren’s in particular, the systemic disease is often diagnosed years after the start of symptoms.53
Overall, we found that the presence of ≥2 aDC in the central cornea was more common in individuals with a diagnosed systemic immune disease, with a sensitivity of 60% and a specificity of 77%. This finding was mostly driven by secondary Sjögren’s. While prior studies did not specifically comment on aDC number, the mean overall DC number in our population of individuals with a systemic immune condition (52.5±62.5 cells/mm2) was similar to that previously described in Sjögren’s (49.0±12.9 to 239.6±52.9 cells/mm2),22, 54 rheumatoid arthritis (25.9±26.6 to 68.2±72.3 cells/mm2),21, 28 ankylosing spondylitis55 (75.5 [51.2–112.6] cells/mm2), and thyroid-induced ophthalmopathy (47.5±38.6 to 76.4±67.8 cells/mm2).27
Interestingly, we also found that Black individuals were more likely to have ≥2 aDC as compared to White individuals. The reason behind this association is uncertain, but previous studies have reported that Black individuals have higher serum levels of inflammatory markers, such as IL-6 and CRP, compared to their White counterparts. 56–59 Interestingly, a more robust proinflammatory state is thought to be a contributing factor to the higher COVID-19 hospitalization and mortality rates seen in Black individuals as compared to White individuals.59– 61 Furthermore, certain nerve parameters were also associated with aDC number, namely increased nerve fiber area and fractal dimension. Other studies in diverse populations have also noted that increased aDC number in individuals with vs without DE was oftentimes accompanied by a higher nerve tortuosity grade (2.30±0.4 to 3.18±0.75 vs 1.09±0.54 to 1.50±0.52),21, 62–64 and nerve beading (323±63 to 387±62 beadings/100μm vs 182±63 to 198±65 beadings/100μm).62, 65 Nerves and DCs likely continuously interact and communicate with one another as was nicely demonstrated in mice, where corneal nerve regeneration after epithelial debridement was significantly impaired in corneas depleted of DCs compared to controls.66 Interestingly, the addition of topical ciliary neurotrophic factor (normally secreted by DCs) restored nerve regeneration in DC depleted corneas after epithelial debridement.66 Moreover, DC depleted corneas of mice exposed to desiccating stress had reduced paracentral corneal nerve density and reduced levels of neurotrophic factors, such as nerve growth factor, substance P and calcitonin gene related peptide, compared to non-DC depleted corneas67, further suggesting an association between nerve health and corneal DCs.
Fortunately, independent of the presence of a systemic immune condition, aDC number decreased with topical anti-inflammatory therapy. This is in agreement with prior studies. In one study of 50 individuals with DE (TBUT <10s, Schirmer’s <10mm/5min), DC numbers decreased after four weeks of therapy with topical loteprednol (61.2±16.7 to 46.5±13.3 cells/mm2).68 Similar data was noted in individuals with Sjögren’s treated with topical cyclosporine for six months (250±108 to 93±58 cells/mm2). Interestingly, DC numbers have also been found to decrease when systemic treatment with prednisone and methotrexate was initiated in individuals with rheumatoid arthritis and secondary Sjögren’s (85.34±61.72 to 24.86±26.25 cells/mm2).21 While we do not have natural history data on the effect of persistent aDCs in the cornea, their presence is probably deleterious to the ocular surface health. In mice exposed to desiccating stress, depletion of APCs in the conjunctiva prevented the accumulation of infiltrating CD4+ T- cells within the ocular surface and preserved the number of goblet cells in the conjunctiva, suggesting that the inflammatory process seen in DE is dependent of APCs.11 In humans, increased aDC number was associated with decreased conjunctival goblet cells number in individuals with Sjögren’s associated DE.69 Given their involvement in inflammation and T-cell activation, there is a biologic plausibility that reducing aDC number in the cornea may have a beneficial long-term effect on the ocular surface health.
Several potential limitations should be noted when interpreting the results of this study. First, this study evaluated a population of older male US veterans seeking eye care services, and thus the findings may not be generalizable to other populations. Second, given the cross-sectional nature of this study, it is not possible to attribute causation, such as when considering relationships between DCs and nerve parameters. Third, DC “activation” status was evaluated morphologically and not using specific markers. However, IHC requires tissue which is not available in most individuals being evaluated for DE. Fourth, there are likely individuals in the group with an undiagnosed systemic immune disease and this would affect our sensitivity and specificity values. Moreover, information on the non-ocular clinical status of individuals with systemic immune diseases were not examined in this study. Fifth, our aDC count was performed manually on two-dimensional and as such, aDCs with dendrites positioned within the Z-axis may not have been counted. We chose this approach despite this limitation as we wanted to examine a strategy that could be immediately implemented in the clinical setting without the need for postimaging processing. Fortunately, we demonstrated good inter-reader reliability in counting aDCs. The implementation of a built-in automated software to count aDCs would standardize this metric across centers and populations. Finally, only a limited number of individuals were started on a topical anti-inflammatory agent and underwent a repeat scan, and we do not have a comparable control group. Thus, we cannot comment on the natural history of change in aDC number in the central cornea.
Despite these limitations, our data suggest that aDC number could be a useful biomarker for a systemic immune disease in individuals with DE symptoms. While our current sensitivity and specificity are still not optimal for a diagnostic test, standardization of image interpretation with development of automated software could improve the numbers and facilitate the use of this test in the clinical setting. However, further longitudinal research is needed to validate our findings in more diverse populations, to confirm the effect of topical anti-inflammatories on aDC numbers and to evaluate how changes in aDCs number relate to changes in clinical symptoms and signs of disease.
Ethical statement.
This study was approved by the Institutional Review Board (IRB) at the Miami Veterans Administration Medical Center and was conducted in accordance with the principles of the Declaration of Helsinki and the United States Health Insurance Portability and Accountability Act.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional), Consejo Nacional de Ciencia y Tecnología (CONACYT) CVU810654 (H. Levine)
Footnotes
Declarations of Interest: None.
Disclaimer: The views expressed in this work are not an official position of the Veterans Health Administration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. American journal of ophthalmology 2017;182:90–8. [DOI] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf 2017;15(3):276–83. [DOI] [PubMed] [Google Scholar]
- 3.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2):75–92. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Alves M, Bunya VY, et al. Tfos dews ii epidemiology report. The ocular surface 2017;15(3):334–65. [DOI] [PubMed] [Google Scholar]
- 5.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. American journal of ophthalmology 2007;143(3):409–15. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Current ophthalmology reports 2013;1(2):51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh J, Shin J-S. The role of dendritic cells in central tolerance. Immune network 2015;15(3):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamrah P, Dana MR. Corneal antigen-presenting cells. Immune Response and the Eye: Karger Publishers, 2007; v. 92. [DOI] [PubMed] [Google Scholar]
- 9.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Investigative ophthalmology & visual science 2003;44(2):581–9. [DOI] [PubMed] [Google Scholar]
- 10.Jiao H, Naranjo Golborne C, Dando SJ, et al. Topographical and morphological differences of corneal dendritic cells during steady state and inflammation. Ocular immunology and inflammation 2020;28(6):898–907. [DOI] [PubMed] [Google Scholar]
- 11.Schaumburg CS, Siemasko KF, De Paiva CS, et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. The Journal of Immunology 2011;187(7):3653–62. [DOI] [PubMed] [Google Scholar]
- 12.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise the Cogan lecture. Investigative ophthalmology & visual science 2004;45(3):722–7. [DOI] [PubMed] [Google Scholar]
- 13.Forrester JV, Xu H, Kuffova L, et al. Dendritic cell physiology and function in the eye. Immunological reviews 2010;234(1):282–304. [DOI] [PubMed] [Google Scholar]
- 14.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Progress in retinal and eye research 2012;31(3):271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Archives of ophthalmology 2012;130(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras-Ruiz L, Regenfuss B, Mir FA, et al. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjögren’s syndrome. PloS one 2013;8(9):e75937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Seminars in ophthalmology: Taylor & Francis, 2010; v. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fea AM, Aragno V, Testa V, et al. The Effect of Autologous Platelet Lysate Eye Drops: An In Vivo Confocal Microscopy Study. Biomed Res Int 2016;2016:8406832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tepelus TC, Chiu GB, Huang J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol 2017;255(9):1771–8. [DOI] [PubMed] [Google Scholar]
- 20.Villani E, Magnani F, Viola F, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci 2013;90(6):576–86. [DOI] [PubMed] [Google Scholar]
- 21.Villani E, Galimberti D, Del Papa N, et al. Inflammation in dry eye associated with rheumatoid arthritis: cytokine and in vivo confocal microscopy study. Innate Immun 2013;19(4):420–7. [DOI] [PubMed] [Google Scholar]
- 22.Lin H, Li W, Dong N, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci 2010;51(1):122–8. [DOI] [PubMed] [Google Scholar]
- 23.Machetta F, Fea AM, Actis AG, et al. In vivo confocal microscopic evaluation of corneal langerhans cells in dry eye patients. Open Ophthalmol J 2014;8:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu J, Chou Y, Hao R, et al. Evaluation of ocular surface impairment in meibomian gland dysfunction of varying severity using a comprehensive grading scale. Medicine (Baltimore) 2019;98(31):e16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qazi Y, Kheirkhah A, Blackie C, et al. In vivo detection of clinically non-apparent ocular surface inflammation in patients with meibomian gland dysfunction-associated refractory dry eye symptoms: a pilot study. Eye (Lond) 2015;29(8):1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villani E, Canton V, Magnani F, et al. The aging Meibomian gland: an in vivo confocal study. Invest Ophthalmol Vis Sci 2013;54(7):4735–40. [DOI] [PubMed] [Google Scholar]
- 27.Wu LQ, Cheng JW, Cai JP, et al. Observation of Corneal Langerhans Cells by In Vivo Confocal Microscopy in Thyroid-Associated Ophthalmopathy. Curr Eye Res 2016;41(7):927–32. [DOI] [PubMed] [Google Scholar]
- 28.Marsovszky L, Resch MD, Nemeth J, et al. In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis. Innate Immun 2013;19(4):348–54. [DOI] [PubMed] [Google Scholar]
- 29.Mastropasqua L, Nubile M, Lanzini M, et al. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. Am J Ophthalmol 2006;142(5):736–44. [DOI] [PubMed] [Google Scholar]
- 30.Resch MD, Marsovszky L, Nemeth J, et al. Dry eye and corneal langerhans cells in systemic lupus erythematosus. Journal of Ophthalmology 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, et al. In vivo confocal microscopic evaluation of corneal nerve fibers and dendritic cells in patients with Behcet’s disease. Frontiers in neurology 2018;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer WJ, Mackert MJ, Kranebitter N, et al. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Current eye research 2012;37(11):1012–8. [DOI] [PubMed] [Google Scholar]
- 33.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Investigative ophthalmology & visual science 2011;52(8):5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg ME, Tervo TM, Müller LJ, et al. In vivo confocal microscopy after herpes keratitis. Cornea 2002;21(3):265–9. [DOI] [PubMed] [Google Scholar]
- 35.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens and Anterior Eye 2010;33(2):55–60. [DOI] [PubMed] [Google Scholar]
- 36.Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Archives of ophthalmology 2000;118(5):615–21. [DOI] [PubMed] [Google Scholar]
- 37.Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the neuropathic pain symptom inventory. Pain 2004;108(3):248–57. [DOI] [PubMed] [Google Scholar]
- 38.Farhangi M, Feuer W, Galor A, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain 2019;160(7):1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640–50. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain® 2011;152(10):2399–404. [DOI] [PubMed] [Google Scholar]
- 41.Dabbah MA, Graham J, Petropoulos IN, et al. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Medical image analysis 2011;15(5):738–47. [DOI] [PubMed] [Google Scholar]
- 42.Dehghani C, Pritchard N, Edwards K, et al. Fully automated, semiautomated, and manual morphometric analysis of corneal subbasal nerve plexus in individuals with and without diabetes. Cornea 2014;33(7):696–702. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Graham J, Petropoulos IN, et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Investigative Ophthalmology & Visual Science 2018;59(2):1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lagali NS, Badian RA, Liu X, et al. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Scientific reports 2018;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coll J, Anglada J, Tomas S, et al. High prevalence of subclinical Sjögren’s syndrome features in patients with autoimmune thyroid disease. The Journal of rheumatology 1997;24(9):1719–24. [PubMed] [Google Scholar]
- 46.Gilboe I, Kvien T, Uhlig T, Husby G. Sicca symptoms and secondary Sjögren’s syndrome in systemic lupus erythematosus: comparison with rheumatoid arthritis and correlation with disease variables. Annals of the rheumatic diseases 2001;60(12):1103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wangkaew S, Kasitanon N, Sivasomboon C, et al. Sicca symptoms in Thai patients with rheumatoid arthritis, systemic lupus erythematosus and scleroderma: a comparison with age- matched controls and correlation with disease variables. Asian Pacific journal of allergy and immunology 2006;24(4):213. [PubMed] [Google Scholar]
- 48.Haga HJ, Naderi Y, Moreno AM, Peen E. A study of the prevalence of sicca symptoms and secondary S jogren’s syndrome in patients with rheumatoid arthritis, and its association to disease activity and treatment profile. International journal of rheumatic diseases 2012;15(3):284–8. [DOI] [PubMed] [Google Scholar]
- 49.Zlatanovic G, Veselinovic D, Cekic S, et al. Ocular manifestation of rheumatoid arthritisdifferent forms and frequency. Bosnian journal of basic medical sciences 2010;10(4):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akpek EK, Klimava A, Thorne JE, et al. Evaluation of patients with dry eye for presence of underlying Sjögren’s syndrome. Cornea 2009;28(5):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta A, Sadeghi PB, Akpek EK. Occult thyroid eye disease in patients presenting with dry eye symptoms. American journal of ophthalmology 2009;147(5):919–23. [DOI] [PubMed] [Google Scholar]
- 52.Henrich CF, Ramulu PY, Akpek EK. Association of dry eye and inflammatory systemic diseases in a tertiary care-based sample. Cornea 2014;33(8):819–25. [DOI] [PubMed] [Google Scholar]
- 53.Akpek EK, Mathews P, Hahn S, et al. Ocular and systemic morbidity in a longitudinal cohort of Sjögren’s syndrome. Ophthalmology 2015;122(1):56–61. [DOI] [PubMed] [Google Scholar]
- 54.Kheirkhah A, Darabad RR, Cruzat A, et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Investigative ophthalmology & visual science 2015;56(12):7179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsovszky L, Nemeth J, Resch MD, et al. Corneal Langerhans cell and dry eye examinations in ankylosing spondylitis. Innate Immunity 2014;20(5):471–7. [DOI] [PubMed] [Google Scholar]
- 56.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a biethnic population. Ethnicity & disease 2011;21(2):142. [PMC free article] [PubMed] [Google Scholar]
- 57.Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clinical chemistry 2008;54(6):1027–37. [DOI] [PubMed] [Google Scholar]
- 58.Walston JD, Fallin MD, Cushman M, et al. IL-6 gene variation is associated with IL-6 and C-reactive protein levels but not cardiovascular outcomes in the Cardiovascular Health Study. Human genetics 2007;122(5):485–94. [DOI] [PubMed] [Google Scholar]
- 59.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. New England Journal of Medicine 2020;382(26):2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vepa A, Bae JP, Ahmed F, et al. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020;14(5):1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phillips N, Park I-W, Robinson JR, Jones HP. The perfect storm: COVID-19 health disparities in US Blacks. Journal of racial and ethnic health disparities 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Castillo JMBt, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Investigative ophthalmology & visual science 2004;45(9):3030–5. [DOI] [PubMed] [Google Scholar]
- 63.Villani E, Galimberti D, Viola F, et al. The cornea in Sjögren’s syndrome: an in vivo confocal study. Investigative ophthalmology & visual science 2007;48(5):2017–22. [DOI] [PubMed] [Google Scholar]
- 64.Tuominen IS, Konttinen YT, Vesaluoma MH, et al. Corneal innervation and morphology in primary Sjögren’s syndrome. Investigative ophthalmology & visual science 2003;44(6):2545–9. [DOI] [PubMed] [Google Scholar]
- 65.Villani E, Galimberti D, Viola F, et al. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Investigative ophthalmology & visual science 2008;49(2):560–4. [DOI] [PubMed] [Google Scholar]
- 66.Gao N, Yan C, Lee P, et al. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. The Journal of clinical investigation 2016;126(5):1998–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi EY, Kang HG, Lee CH, et al. Langerhans cells prevent subbasal nerve damage and upregulate neurotrophic factors in dry eye disease. PloS one 2017;12(4):e0176153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villani E, Garoli E, Termine V, et al. Corneal confocal microscopy in dry eye treated with corticosteroids. Optometry and Vision Science 2015;92(9):e290–e5. [DOI] [PubMed] [Google Scholar]
- 69.Pflugfelder SC, Bian F, Gumus K, et al. Severity of Sjögren’s syndrome keratoconjunctivitis sicca increases with increased percentage of conjunctival antigen-presenting cells. International journal of molecular sciences 2018;19(9):2760. [DOI] [PMC free article] [PubMed] [Google Scholar]