Abstract
Despite immense strides in therapeutic advances, clinical outcomes continue to be less than ideal for people with type 1 diabetes. This discrepancy has prompted an outpouring of quality improvement (QI) initiatives to address the medical, psychosocial, and health equity challenges that complicate ideal type 1 diabetes care and outcomes. This article reviews a framework for QI in diabetes care that guided the development of the T1D Exchange Quality Improvement Collaborative to improve care delivery and health outcomes in type 1 diabetes. Evaluation of the methodology, outcomes, and knowledge gained from these initiatives will highlight the importance of continued QI initiatives in diabetes care.
Type 1 diabetes is characterized by immune-mediated depletion of pancreatic β-cells, resulting in lifelong dependence on insulin. Among the U.S. population, an estimated 187,000 youths and 1.4 million adults have type 1 diabetes (1). This prevalence is further intensified by the impact of the burden of disease as related to its complications, excess mortality, and challenges in access and affordability of insulin (2). The life expectancy for individuals with type 1 diabetes is estimated to be 3.6 years less than the general population (3).
Since the Diabetes Control and Complications Trial provided irrefutable evidence for the benefit of optimal glycemic control in mitigating risks of long-term micro- and macrovascular complications (4–8), many important advances in diabetes therapy have been made. However, youths and adults with type 1 diabetes continue to struggle to meet the glycemic targets outlined by the American Diabetes Association (ADA) (9,10), with just 17% of youths in the TID Exchange clinic registry achieving an A1C <7.5% and 21% of adults having an A1C <7.0% between 2016 and 2018 (11). Even more concerning, the adjusted mean A1C increased by 0.6% from 2010–2012 to 2016–2018 (11).
The discrepancy between therapeutic innovations and clinical outcomes is likely the result of ongoing gaps in care delivery, psychosocial needs, self-management, health system design, and equity of care. A 2016 meta-analysis (12) highlighted a lack of high-quality, well-designed interventions to improve clinical and psychosocial outcomes in type 1 diabetes. The awareness of this incongruence in care advancement and patient outcomes has prompted a surge of quality improvement (QI) initiatives to address the medical challenges, as well as the equally significant psychosocial aspects of type 1 diabetes, including diabetes distress, depression, anxiety, disordered eating behaviors, and diabetes-related family conflict (13).
QI methods are systematic and continuous actions that lead to measurable improvement in health care services and the health status of targeted patient groups (14). In turn, the implementation of QI methodologies provides reliable application of evidence-based care. In this review, we discuss QI projects in the United States that have been implemented to improve care delivery and health outcomes in type 1 diabetes. Evaluation of the methodology and knowledge gained from these initiatives will highlight the importance of continued QI initiatives in diabetes care, which will ultimately improve clinical outcomes, reduce psychosocial burden, and improve health-related quality of life.
A Framework for QI in Diabetes Care
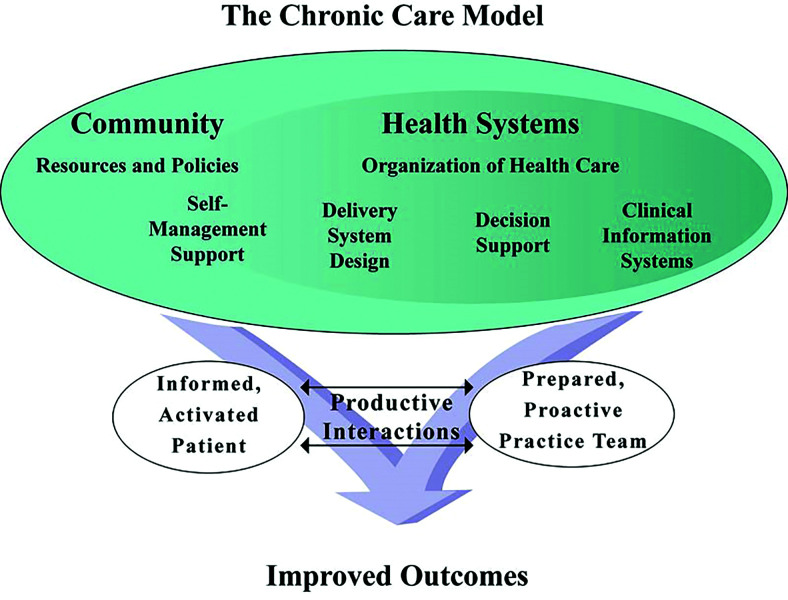
Two decades ago, the development of the Chronic Care Model (CCM) outlined a systematic approach to improving health care delivery (15). The CCM emphasizes regular, patient-centered interactions between individuals and their health care team. It focuses on improving psychosocial support and leveraging information technology to redesign health systems for better care delivery. The model emphasizes four main categories of interventions: 1) self-management support, 2) delivery system design, 3) decision support, and 4) clinical information systems (Figure 1) (16). When integrated, these elements produce better interactions between patients and care teams (17).
FIGURE 1.
The Chronic Care Model. Developed by the MacColl Institute. ©ACP-ASIM Journals and Books (16).
The Institute for Healthcare Improvement (IHI) Breakthrough Series Collaborative further developed the CCM by developing strategies to serially test and adapt QI interventions to generate a change in outcomes. IHI expanded on this concept by promoting a Triple Aim initiative to improve population health, reduce health care costs, and improve patient satisfaction (18). IHI’s Breakthrough Series provided a framework for collaborative improvement that catalyzed the emergence of partnerships worldwide to apply QI methodologies to the diabetes population (19–21). Groups such as the Diabetes Quality Improvement Program have applied the IHI Triple Aim model to focus on improving care delivery, as measured by A1C and end-organ complications (22,23).
The Institute of Medicine (IOM) cultivated improvement science further to the development of a Learning Health System model (24). In this model, patients and families are key members of the health care team, which promotes ideal, patient-centered care. The Collaborative Chronic Care Network is an example of a learning health system that was originally focused on inflammatory bowel disease and cystic fibrosis and has served as a model for QI initiatives in type 1 diabetes (25).
Initially, these models provided the context for the development of diabetes QI initiatives, primarily in the realm of type 2 diabetes. Initial diabetes-related interventions included implementation of multidisciplinary teams, formation of patient registries, better dissemination of information, continuation of QI work, and development of patient education initiatives (26,27). The framework provided by the CCM, IHI, and IOM, and the experience of type 2 diabetes QI interventions, were instrumental in applying improvement science to type 1 diabetes care. This effort has included the identifying metrics to measure processes and outcomes, developing QI interventions, and using Plan-Do-Study-Act (PDSA) cycles to analyze the success of the interventions for possible full implementation or to develop another change cycle (28). The focus on improved population health also prompted the development of large clinical registries to promote shared communication among health systems (29).
T1D Exchange Quality Improvement Collaborative
Quality improvement collaboratives (QICs) enable an organized, multifaceted, multidisciplinary approach that includes teams from multiple health care institutions uniting together to develop, apply, and disseminate QI initiatives for a given health care topic. QICs are supported by faculty experts in the given topic area who identify best clinical practices and facilitate implementation strategies to improve care. Teams apply QI methods locally, undertake rapid PDSA cycles to understand tests of change, and share data, novel interventions, and lessons learned with QIC partner institutions. Ideally, this process results in more effective implementation and spread of QI interventions and allows benchmarking of local progress to other sites. In a recent systematic review (30), QICs were reported as yielding significant improvements in target clinical processes and outcomes across hospital-based and ambulatory settings. The QIC model for shared learning, reporting, and benchmarking outcomes in QI interventions prompted the development of the T1D Exchange Quality Improvement Collaborative (T1DX-QI), which focuses on type 1 diabetes.
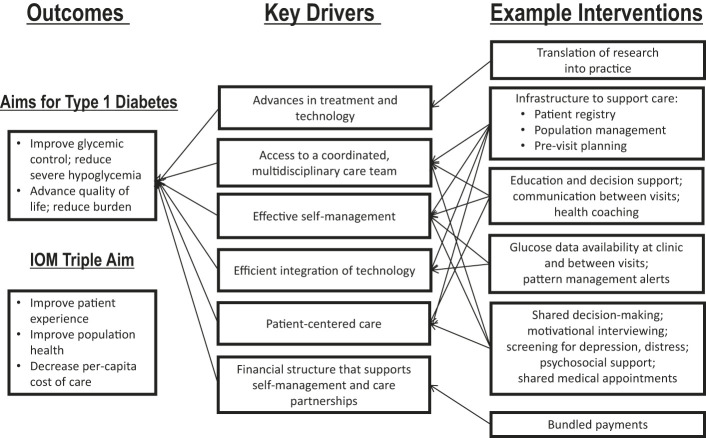
In brainstorming the design of the T1DX-QI, Corathers et al. (29) proposed a learning health system based on the aforementioned improvement science principles. They developed a key driver diagram that focused on improving glycemic control, reducing severe hypoglycemic events, improving health-related quality of life, and decreasing the psychosocial burden of diabetes (Figure 2) (29). To achieve these aims, the T1DX-QI initially concentrated on drivers such as self-management, integration of technologies, and patient-centered care (29). Furthermore, they emphasized the need for future models that are cooperative—not only among patients, families, and the health care team, but also among health systems, with encouraged sharing of information and collaboration.
FIGURE 2.
Key driver diagram for type 1 diabetes (29).
In developing the T1DX-QI, a multidisciplinary team consisting of patients, family members, clinicians, informaticians, computer scientists, software engineers, educators, and experts in community integration and business was formed to coproduce an optimal type 1 diabetes care process model for improved health outcomes (31,32). Ethnographic interviews were conducted at two large health systems with a diverse group of participants, which included people with type 1 diabetes, caregivers, physicians, nurse educators, social workers, dietitians, and health care administrators with an interest in diabetes, to characterize the barriers that patients and families commonly encounter (31). The team also used extensive medical literature queries and invited expert guidance. These various explorations revealed that the current system of diabetes care was failing in multiple aspects: cost, psychosocial support, reliability, and translatability (31). Subsequently, with the additional insight of patient- and family-specific barriers from the interviews, the team developed 84 intervention ideas. These were narrowed down using an impact-effort matrix to rank the interventions based on their perceived impact and feasibility, which resulted in a robust set of ideas with potential for having breakthrough impact on research, care delivery, and health outcomes in type 1 diabetes.
As a multicenter initiative, the T1DX-QI aims to accelerate QI interventions to improve the care of people with type 1 diabetes through shared learning and continuous review of best practices. At the launch of the collaborative, 10 large U.S. diabetes centers participated, including seven pediatric clinics and three adult clinics collectively serving >24,000 patients with type 1 diabetes (31). The initial clinics were selected based on their baseline QI capacity, number and expertise of patients or family members in improvement processes, and information technology landscape. Clinic personnel from each site received formal QI training and were then regularly evaluated with an adapted Quality Improvement Organizational Readiness Assessment that reviewed specific QI domains, including team structure, foundation, capacity or proficiency, and success (31). This tool uses a simple “yes”/”no” scoring system, which allowed the staff to determine which of the aforementioned domains are insufficient and subsequently provide resources to strengthen the targeted areas. Within 6 months of training, 80% of clinics achieved scores of ≥75% on the readiness assessment. Teams were structured to include a clinical champion, a QI coordinator, a senior department leader, a data analyst, and a patient/family representative. Collaboration among all clinic sites (virtually, in-person, and via electronic database) and formation of patient/parent advisory boards were encouraged to allow for continued sharing of data and ideas (31).
Focused Interventions in Type 1 Diabetes Care
With the maturation of the T1DX-QI, multiple QI interventions in diabetes care have been deployed and evaluated across several areas. The bulk of these QI initiatives have focused on three key areas: care delivery, self-management, and psychosocial support.
Care Delivery
Improving the delivery of diabetes care includes a focus on risk-stratification tools, pre-visit planning, access to care, and a multidisciplinary team approach to care. An example of improvement science to enhance diabetes care delivery was the development of the Type 1 Diabetes Care Index (T1DCI) (33), a metric developed by Nationwide Children’s Hospital (NCH) to aggregate missed opportunities to deliver elements of optimal diabetes care based on ADA (9) and International Society for Pediatric and Adolescent Diabetes (34) guidelines. NCH used the T1DCI metric to identify gaps in care and focus QI efforts. Implementation of the T1DCI guided QI interventions in processes for care delivery, which led to a 26% improvement in T1DCI scores over 12 months at NCH (33). The same QI team at NCH also created the Type 1 Diabetes Composite Score (T1DCS) as a clinical indicator that goes beyond A1C to comprehensively reflect patient status. The T1DCS aggregates nine outcome measures from the electronic health record (EHR) associated with optimal diabetes care and assigns a score for each patient, with higher scores reflecting better management and outcomes. In using the T1DCS to direct QI and clinical care, the NCH team observed a right shift in scores, indicating improved clinical outcomes and compliance with guidelines (35).
A pre-visit planning work group involving five T1DX-QI clinics improved information collection by 91%, including asking patients/families about their needs and goals, reviewing appropriate screening tests, and ensuring that refills were completed (31). High-risk patients, which T1DX-QI defines as those having two A1C values >9% in the preceding 12 months, have an increased risk of acute and long-term complications. In a QI project to reduce the proportion of patients who fell into the high-risk category, 10 centers deployed multiple PDSA cycles, resulting in a decrease of 3 percentage points, from a baseline of 40% to 37%, over a 15-month period (36). Pediatric and adult clinics co-developed the project design to support patient needs based on five key driver interventions: 1) glucose monitoring, 2) insulin management, 3) patient-centered care, 4) access to clinical care, and 5) psychosocial care. Successful interventions included using a patient navigator to reach out to families between visits, increasing depression screening, developing classes to encourage patients to be quicker to accept continuous glucose monitoring (CGM), and addressing social determinants of health (SDOH). In an effort to assess patients at risk for an acute complication, one T1DX-QI clinic developed an EHR-based, automated tool to stratify risk of DKA (unpublished data, Texas Children’s Hospital). This provider-facing tool correctly determined risk in 75% of patients based on subsequent DKA outcomes. QI and clinical interventions to prevent DKA for those at high risk are currently underway.
Self-Management
Effective patient self-management strategies have been associated with improved glycemic control (37–40). One T1DX-QI center developed a score based on six diabetes self-management habits, including checking glucose ≥4 times/day or using CGM, administering three or more insulin boluses daily, using an insulin pump, administering bolus insulin doses before meals, reviewing diabetes data between visits, and changing insulin doses between visits. A total habit score was created by summing all six habits based on EHR documentation. A1C was lower for patients performing the self-management habits, with a reduction of 0.7% percentage points in per 1-unit increase in habit score (41). These metrics will be adopted by multiple T1DX-QI centers to support interventions to improve diabetes self-management and glycemic outcomes.
Use of diabetes devices, including CGM systems and insulin pumps, has the potential for improving glycemic control and improving quality of life. Ten clinics in the T1DX-QI participated in a project aimed at increasing CGM use in patients aged 12–26 years. Eight of 10 centers saw improved CGM uptake, with an overall 12% increase of CGM use, from a baseline of 36% to 48%, over a 20-month period across the entire T1DX-QI cohort (42). Initiatives to improve CGM use included investing in new staff roles to support CGM uptake and creating patient navigator positions dedicated to helping patients navigate insurance coverage, industry forms, and CGM training sessions. In another collaborative project focused on diabetes devices, five T1DX-QI sites deployed QI interventions to expand insulin pump use, with a 10% overall increase from a baseline of 46 to 56% for the entire cohort (43). Successful interventions to expand pump use included redesigning clinic workflows, developing mobile technology classes, and coaching patients to take insulin before meals.
Psychosocial Support
Psychosocial stresses such as family conflict, underlying mood disorders, and diabetes distress contribute to poor health outcomes in patients with type 1 diabetes. In adolescents with type 1 diabetes, negative mood and feelings of ineffectiveness have been closely correlated with decreased frequency of blood glucose monitoring and higher A1C (44).
In a QI project to expand depression screening, six T1DX-QI centers developed initiatives to increase consistent referral and screening and to increase psychosocial resources with the use of health information technology (45). By implementing bimonthly calls and three learning sessions focused on depression screening, they saw an increase in screening of >60%, from a baseline of 10% to 71% across all sites. Notably, 7.8% of patients across all sites had a positive depression screen, thus allowing further evaluation and treatment. Comparison of psychosocial screening scores across sites is limited by the use of different instruments and cut-off scores to define a positive screen, so the T1DX-QI is moving toward standardizing depression screening across sites, which might enable such analyses.
Another T1DX-QI clinic similarly aimed to address this psychosocial gap of effective recognition of underlying mood disorders to improve diabetes care delivery. This was accomplished by expanding depression screening via updates to their health information technology to allow for automation of screening dissemination, data capture, measures of progress, and the referral process. Implementation of these initiatives resulted in a 75% improvement in screening rates using the Patient Health Questionnaire [PHQ]-2 and PHQ-9 and allowed >89% of patients with a positive screen to meet with a social worker for a targeted mental health assessment, counseling, referral to local resources, and/or safety planning before leaving the clinic (46).
Role of QI in Addressing SDOH
SDOH are conditions in the environment in which people live that affect a wide range of health and quality-of-life outcomes. Five key aspects of SDOH identified by Healthy People 2020 include 1) economic stability, 2) education, 3) social and community context, 4) health and health care, and 5) neighborhood and built environment (47). Disparities in any of these areas can lead to inequities in health outcomes. Diabetes outcomes as measured by glycemic control and number of acute complications, use of technology, and access to care are all worse in the Non-Hispanic Black and Hispanic populations (48). The barriers for these populations may be the result of differences in language or culture, lack of financial resources, or considerable distances to access care (49). Several QI interventions aimed at reducing health inequities among patients with type 2 diabetes have been deployed in the primary care setting, and evidence suggests that some interventions can improve diabetes-related health outcomes in socially disadvantaged populations (50). In a systematic review of diabetes QI interventions, Lu et al. (49) expressed concern that QI strategies designed for the general population may not be accessible to or have the same efficacy in disadvantaged groups.
In a recent perspectives article, T1DX-QI members described how QI tools and principles can be adapted into a framework for advancing health equity. To address the literature gap on practical ways health care providers can address inequities in diabetes, the authors proposed a 10-step framework for addressing structural and systemic racism, economic disparities, and education inequities in diabetes care delivery. The framework relies on data to identify SDOH, engage an equitable project team with shared decision-making power, develop policies to expand access to care for the most vulnerable patients, and train clinic staff by naming structural racism as a driver of health inequities (48). Strategies to incorporate this health equity framework across T1DX-QI member organizations are currently underway.
Economic Value of QI Interventions in Type 1 Diabetes
With increasingly constrained health care budgets, QI initiatives must have feasible economic value to be sustained in the long term. Emerging evidence suggests that QICs such as the T1DX-QI have potential to yield cost savings to the health care system, but more rigorous cost-effectiveness studies are needed (51). QI interventions specific to diabetes care may be cost-effective in leading to declines in health care utilization with improved glycemic control (52). This effect may not be experienced as a direct financial benefit to hospital systems or clinical practices but could have a large impact in value-based care programs.
Conclusion
Type 1 diabetes has garnered much attention in the realm of clinical research and advancement of technology in the past few decades; however, major gaps in health outcomes persist. These disparities may be the result of the mismatch between evidence and care delivery, discrepancies between the potential and reality of burden from current therapies, and racial/ethnic inequalities in access and outcomes. Only recently has diabetes-specific QI science blossomed with a global appreciation of the contributions that health systems, self-management, psychosocial burdens, and health equity have on glycemic control in patients with type 1 diabetes. A continued drive to improve health care delivery and diabetes management for people with type 1 diabetes has helped QI science in the United States progress to the ongoing and encouraging projects being carried out today. The sharing of best practices through the T1DX-QI is anticipated to continue to amplify impact and accelerate improvement in diabetes outcomes, which also has potential economic value.
Limitations of this review include the large proportion of QI work that goes unpublished and the lack of universal application of rigorous QI methodology and outcome-sharing even within robust collaborative networks such as the T1DX-QI. Given the major gaps in type 1 diabetes outcomes, continued evolution in QI methodology and focused QI interventions are essential to advance quality of life and health outcomes for people with type 1 diabetes.
Article Information
Acknowledgments. The authors thank the Leona M. and Harry B. Helmsley Charitable Trust for funding the T1DX-QI. The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within T1DX-QI, who continually seek to improve care and outcomes for people with diabetes.
Duality of Interest
O.E. is a compensated Health Equity Advisory Board member for Medtronic Diabetes and serves as the principal investigator for investigator-led projects sponsored by Abbott, Dexcom, Eli Lilly, Insulet, and Medtronic. J.M.L. is on the medical advisory board for GoodRx. D.J.D. is a consultant to Dexcom and Insulet. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
O.Z.B.G. and D.J.D. developed the concept for this article and wrote the manuscript. O.E. analyzed the data. All authors reviewed/edited the manuscript. O.Z.B.G., O.E., and D.J.D. are guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is part of a special article collection available at https://clinical.diabetesjournals.org/collection/quality-improvement-initiatives-in-type-1-diabetes.
References
- 1. American Diabetes Association . Statistics about diabetes. Available from https://www.diabetes.org/resources/statistics/statistics-about-diabetes. Accessed 27 October 2020
- 2. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DCCT Research Group . Diabetes Control and Complications Trial (DCCT): update. Diabetes Care 1990;13:427–433 [DOI] [PubMed] [Google Scholar]
- 5. Gosmanov AR, Gosmanova EO. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the DCCT/EDIC cohort. Arch Intern Med 2011;171:1596; author reply 1597 [DOI] [PubMed] [Google Scholar]
- 6. Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Genuth SM, Backlund JYC, Bayless M, et al.; DCCT/EDIC Research Group . Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes 2013;62:3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liakishev AA. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. Results of the DCCT/EDIC study. Kardiologiia 2006;46:73 [in Russian] [PubMed] [Google Scholar]
- 9. American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S163–S182 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 [DOI] [PubMed] [Google Scholar]
- 11. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Hara MC, Hynes L, O’Donnell M, et al.; Irish Type 1 Diabetes Young Adult Study Group . A systematic review of interventions to improve outcomes for young adults with type 1 diabetes. Diabet Med 2017;34:753–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Department of Health and Human Services, Health Resources and Services Administration . Quality Improvement. Bethesda, MD, Health Resources and Services Administration, 2011 [Google Scholar]
- 15. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78 [DOI] [PubMed] [Google Scholar]
- 16. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract 1998;1:2–4 [PubMed] [Google Scholar]
- 17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288:1775–1779 [DOI] [PubMed] [Google Scholar]
- 18. Institute for Healthcare Improvement . The IHI Triple Aim. Available from https://www.ihi.org/Engage/Initiatives/TripleAim/pages/default.aspx. Accessed 2 November 2020
- 19. Kilo CM. A framework for collaborative improvement: lessons from the Institute for Healthcare Improvement’s Breakthrough Series. Qual Manag Health Care 1998;6:1–13 [DOI] [PubMed] [Google Scholar]
- 20. Wagner EH, Glasgow RE, Davis C, et al. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv 2001;27:63–80 [DOI] [PubMed] [Google Scholar]
- 21. Peterson A, Hanberger L, Åkesson K, Bojestig M, Andersson Gäre B, Samuelsson U. Improved results in paediatric diabetes care using a quality registry in an improvement collaborative: a case study in Sweden. PLoS One 2014;9:e97875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Connor PJ, Bodkin NL, Fradkin J, et al. Diabetes performance measures: current status and future directions. Diabetes Care 2011;34:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLaughlin S. The Diabetes Quality Improvement Project. Diabetes Spectr 2000;13:5–8 [Google Scholar]
- 24. Olsen LA, Aisner D, McGinnis JM, Eds. The Learning Healthcare System: Workshop Summary. Washington, D.C., National Academies Press, 2007 [PubMed] [Google Scholar]
- 25. C3N Project . Homepage. Available from http://c3nproject.org. Accessed 2 November 2020
- 26. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet 2012;379:2252–2261 [DOI] [PubMed] [Google Scholar]
- 27. Worswick J, Wayne SC, Bennett R, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews. What does the evidence tell us? Syst Rev 2013;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langley G, Moen R, Nolan K, Nolan T, Norman C, Provost L. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco, CA, Jossey-Bass Publishers, 2009 [Google Scholar]
- 29. Corathers SD, Schoettker PJ, Clements MA, et al. Health-system-based interventions to improve care in pediatric and adolescent type 1 diabetes. Curr Diab Rep 2015;15:91. [DOI] [PubMed] [Google Scholar]
- 30. Wells S, Tamir O, Gray J, Naidoo D, Bekhit M, Goldmann D. Are quality improvement collaboratives effective? A systematic review. BMJ Qual Saf 2018;27:226–240 [DOI] [PubMed] [Google Scholar]
- 31. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes 2020;38:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seid M, Dellal G, Peterson LE, et al. Co-designing a collaborative chronic care network (C3N) for inflammatory bowel disease: development of methods. JMIR Human Factors 2018;5:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Obrynba KS, Indyk JA, Gandhi KK, Buckingham D, Kamboj MK. The Diabetes Care Index: a novel metric to assess delivery of optimal type 1 diabetes care. Pediatr Diabetes 2020;21:637–643 [DOI] [PubMed] [Google Scholar]
- 34. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19(Suppl. 27):105–114 [DOI] [PubMed] [Google Scholar]
- 35. Indyk JA, Buckingham D, Obrynba KS, et al. The Type 1 Diabetes Composite Score: an innovative metric for measuring patient care outcomes beyond hemoglobin A1c. Pediatr Qual Saf 2020;5:e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rioles N, Bunker C, Clements M, et al. Moving the needle on high-risk TID panel: lessons from the T1D Exchange Quality Improvement Collaborative (T1DX-QI) [Abstract]. Diabetes 2020;69:150-LB [Google Scholar]
- 37. Beck RW. Downloading diabetes device data: empowering patients to download at home to achieve better outcomes. Diabetes Technol Ther 2015;17:536–537 [DOI] [PubMed] [Google Scholar]
- 38. Wong JC, Neinstein AB, Spindler M, Adi S. A minority of patients with type 1 diabetes routinely downloads and retrospectively reviews device data. Diabetes Technol Ther 2015;17:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller KM, Beck RW, Bergenstal RM, et al.; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D Exchange clinic registry participants. Diabetes Care 2013;36:2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell MS, Schatz DA, Chen V, et al.; T1D Exchange Clinic Network . A contrast between children and adolescents with excellent and poor control: the T1D Exchange clinic registry experience. Pediatr Diabetes 2014;15:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J, Garrity A, Hirschfeld E, et al. “Six Habits”: quality metrics to support glycemic outcomes in type 1 diabetes. Poster presented at the American Diabetes Association’s virtual 81st Scientific Sessions, 25–29 June 2021 [Google Scholar]
- 42. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across ten national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI) [Abstract]. Diabetes 2020;69:145-LB [Google Scholar]
- 43. Ebekozien O, Rioles N, Indyk JA, et al. Increasing insulin pump use across five national diabetes centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI) [Abstract]. Diabetes 2020;69:146-LB31757794 [Google Scholar]
- 44. McGrady ME, Hood KK. Depressive symptoms in adolescents with type 1 diabetes: associations with longitudinal outcomes. Diabetes Res Clin Pract 2010;88:e35–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Majidi S, Jolly MC, Alonso GT, et al. Incorporating depression screening into diabetes clinics across the T1DX learning collaborative [Abstract]. Diabetes 2018;67:1309-P [Google Scholar]
- 46. Marker AM, Patton SR, McDonough RJ, Feingold H, Simon L, Clements MA. Implementing clinic-wide depression screening for pediatric diabetes: an initiative to improve healthcare processes. Pediatr Diabetes 2019;20:964–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion . Social determinants of health. Available from https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed 18 December 2020
- 48. Ebekozien O, Ori O, Nicole R, Shideh M, Nana-Hawa YJ, Manmohan K. Equitable post-COVID-19 care: a practical framework to integrate health equity in diabetes management. J Clin Outcomes Manag 2020;27:256–259 [Google Scholar]
- 49. Lu JB, Danko KJ, Elfassy MD, Welch V, Grimshaw JM, Ivers NM. Do quality improvement initiatives for diabetes care address social inequities? Secondary analysis of a systematic review. BMJ Open 2018;8:e018826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terens N, Vecchi S, Bargagli AM, et al. Quality improvement strategies at primary care level to reduce inequalities in diabetes care: an equity-oriented systematic review. BMC Endocr Disord 2018;18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de la Perrelle L, Radisic G, Cations M, Kaambwa B, Barbery G, Laver K. Costs and economic evaluations of quality improvement collaboratives in healthcare: a systematic review. BMC Health Serv Res 2020;20:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care 2018;41:985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]