To the Editor:
Current topical medications for rosacea-associated erythema include brimonidine and oxymetazoline, which induce cutaneous vasoconstriction. Topical timolol has been used for infantile hemangioma due to its antiangiogenic and vasoconstrictive effects, but its efficacy for treating rosacea-associated erythema is unknown.
Detailed methods are provided in the supplement. In brief, eight adults with rosacea involving flushing and persistent erythema (Table S1) underwent twice-daily treatment with topical timolol 0.5% gel-forming solution in a split-face study (Fig S1). One side of the face was treated for 16 weeks; the contralateral side received no treatment for eight weeks followed by eight weeks of treatment. We measured facial erythema (a*) with tristimulus colorimetry and computer-aided image analysis (CAIA) of cross-polarized photographs at baseline and every four weeks until four weeks after treatment discontinuation. Participants rated flushing from 1 (“much worse”) to 5 (“much better”) at each post-baseline visit.
Clinical photographs of two participants are shown in Fig S2. Other than one episode of transient lower eyelid sensitivity, no adverse events were reported. No participants reported worsened flushing during treatment; four noted improvement with treatment, while three noted worsening after treatment discontinuation (Fig S3). Colorimeter and CAIA-measured a* were strongly correlated (r=0.71, P<.001, Fig S4). Mixed-effects models showed that erythema decreased on both treated and untreated sides of the face during the first eight weeks but were statistically insignificant (Fig 1, Table S2). When both sides of the face were subsequently treated, there was improvement only on the longer-treated side most significant at week 12 (colorimeter, -20.0%, adjusted P=.001; CAIA, -20.9%, adjusted P=.047), comparable in magnitude to measurements in Fowler et al.’s study of brimonidine for rosacea.1
Figure 1. Change in facial erythema (a*) before and after treatment with topical timolol 0.5% gel-forming solution across all participants.
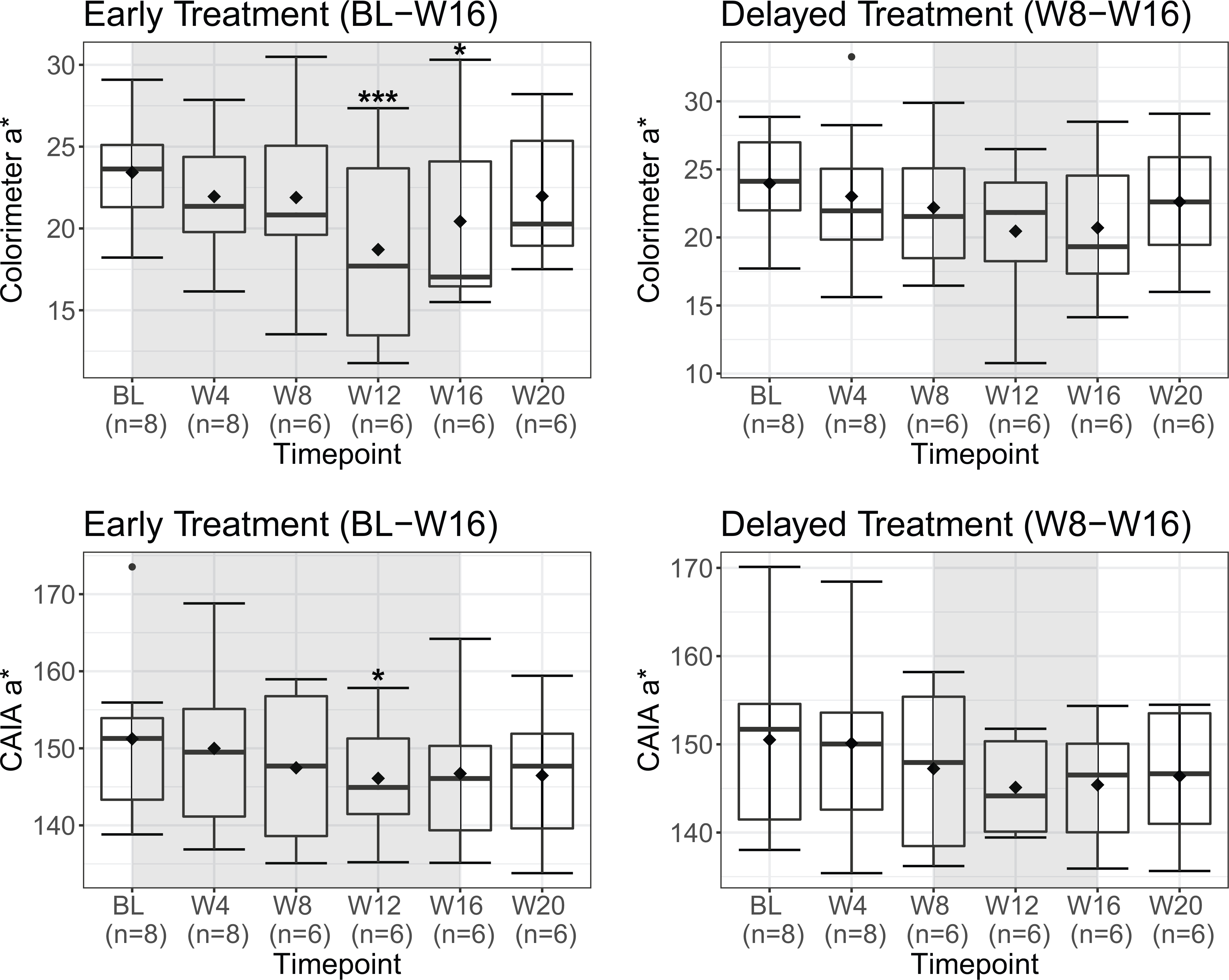
Colorimeter and computer aided image analysis (CAIA)-measured erythema (a*) at each visit for sides of the face assigned to early treatment (baseline – week 16) and delayed treatment (week 8 – week 16). Colorimeter a* measured with Chroma Meter CR-400 at a standardized point of the malar cheek (intersection of the midpupillary line and line drawn laterally from the ipsilateral nasal ala). Computer-aided image analysis (CAIA) a* obtained by converting cross-polarized photographs to 8-bit grayscale a* images with ImageJ software, manually outlining the cheek (infraorbital, zygomatic, and mandibular regions), and measuring average pixel intensity across the region. Both measures of erythema indicate red-green chromaticity with different scales; colorimeter a* may range from-60 (green) to +60 (red), while CAIA a* may range from 0 (green) to 255 (red). Shaded regions on graphs indicate times when participants received treatment. Horizontal band and diamond represent the median and mean respectively; bottom and top of each box, first and third quartiles; lower error bar extends to the lowest data point within 1.5 times the interquartile range from the first quartile; upper error bar extends to the highest data point within 1.5 times the interquartile range from the third quartile. Data points beyond 1.5 times the interquartile range above the third quartile or below the first quartile are plotted individually as outliers. P-values calculated from mixed-effects models using t-tests with Satterthwaite’s method and corrected for multiple comparisons with Benjamini-Hochberg procedure. *adjusted P<.05 compared to baseline, *** adjusted P<.001 compared to baseline. BL, baseline; W4, week 4; W8, week 8; W12, week 12; W16, week 16; W20, week 20.
There was recovery of erythema at week 16 suggestive of tolerance, which has been observed in long-term treatment of glaucoma with timolol, likely from beta-adrenoreceptor upregulation.2 Tolerance to timolol in glaucoma has been reversed with treatment suspension and interim treatment with an alpha-agonist.3 We also observed rebound erythema following discontinuation of timolol, similar to prior reports in patients treated with brimonidine.4,5 Future studies may investigate whether alternating between beta-blockers like timolol and alpha-agonists like brimonidine may mitigate tolerance and rebound erythema associated with either agent.
Our study was confounded by small sample size and potential carryover effects. Many participants showed bilateral improvement of facial erythema during the first eight weeks despite unilateral treatment (Fig S5), possibly reflecting placebo effect or spread of medication through cutaneous vasculature. We tracked adherence with self-reported diaries but did not objectively measure medication application; whether improvement on the treated side may have encouraged timolol use on the contralateral side among participants was unknown. Two participants only completed two visits due to scheduling difficulty, resulting in 17% loss of data analyzed in mixed-effects models, which may have biased our results. As statistically significant improvement was not seen until 12 weeks of treatment in this study, future studies may investigate more potent formulations. More studies on short-term clinical response (hours-days), tolerance, and rebound erythema will help elucidate the suitability of topical timolol for rosacea-associated erythema.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Garza and Mr. Tsai had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/Support:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under R01AR074846 01 and a grant from the National Rosacea Society to LAG. This work was also supported by the Thomas Provost, MD Young Faculty Development Fund of Johns Hopkins Dermatology to LAG.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions:
The authors thank the Cutaneous Translational Research Program (CTReP) at the Johns Hopkins Department Dermatology for organizing human subjects and preparing tissue samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to declare.
IRB approval status: Reviewed and approved by Johns Hopkins University IRB (IRB00074049)
Trial Registration:ClinicalTrials.gov Identifier: NCT02774590
REFERENCES
- 1.Fowler J, Jarratt M, Moore A, et al. Once-daily topical brimonidine tartrate gel 0·5% is a novel treatment for moderate to severe facial erythema of rosacea: results of two multicentre, randomized and vehicle-controlled studies. British Journal of Dermatology. 2012;166(3):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boger WP 3rd. Shortterm "escape" and longterm "drift." The dissipation effects of the beta adrenergic blocking agents. Surv Ophthalmol. 1983;28Suppl:235–242. [DOI] [PubMed] [Google Scholar]
- 3.Gandolfi SA. Restoring sensitivity to timolol after long-term drift in primary open-angle glaucoma. Investigative Ophthalmology & Visual Science. 1990;31(2):354–358. [PubMed] [Google Scholar]
- 4.Ilkovitch D, Pomerantz RG. Brimonidin eeffective but may lead to significant rebound erythema. J Am Acad Dermatol. 2014;70(5):e109–110. [DOI] [PubMed] [Google Scholar]
- 5.Okwundu N, Cline A, Feldman SR. Difference in vasoconstrictors: oxymetazoline vs. brimonidine. J Dermatolog Treat. 2019:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


