Fig. 7.
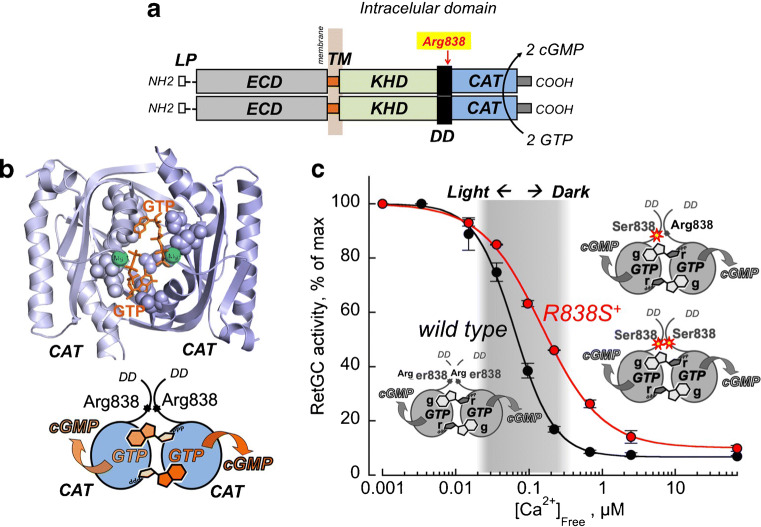
a The diagram of RetGC1 homodimer. Each GUCY2D–coded polypeptide includes the N-terminal leader peptide (LP), “extracellular” domain (ECD), transmembrane region (TM), kinase homology domain (KHD), dimerization domain (DD), and catalytic domain (CAT) [36, 66]. Two RetGC1 subunits in the homodimer catalyze GTP-to-cGMP conversion by the two catalytic domains forming a single active site; the arrow indicates the position of Arg838 frequently substituted in GUCY2D CORD6 alleles [40, 106]. b Top panel. The three-dimensional model of the dimerized RetGC1 catalytic domains making a single active site for binding simultaneously two Mg2+ GTP substrate molecules (Liu et al. [65]). The two GTP molecules are highlighted in orange and the two Mg2+ counterions in green. The schematic in the bottom panel illustrates that each of the two GTP molecules in the active site is simultaneously held by both RetGC1 subunits; none of the subunits can bind GTP on its own. c CORD6 mutation, Arg838Ser, reduces Ca2+ sensitivity of both homodimer Ser838:Ser838 [86, 102] and heterodimer Arg8383:Ser838s [98]; therefore, in transgenic mice expressing both wild type and Arg838Ser RetGC1 (red), RetGC remains active outside the physiological range of free Ca2+ in the dark (shaded in gray) [30, 105] (the data in the graph are from Dizhoor et al. (2016), article in Journal of Biological Chemistry [30])