Fig. 7. A schematic model of SNORD50A/B inducing ubiquitin-proteasome degradation of wild-type p53 by promoting the interaction between TRIM21 and GMPS.
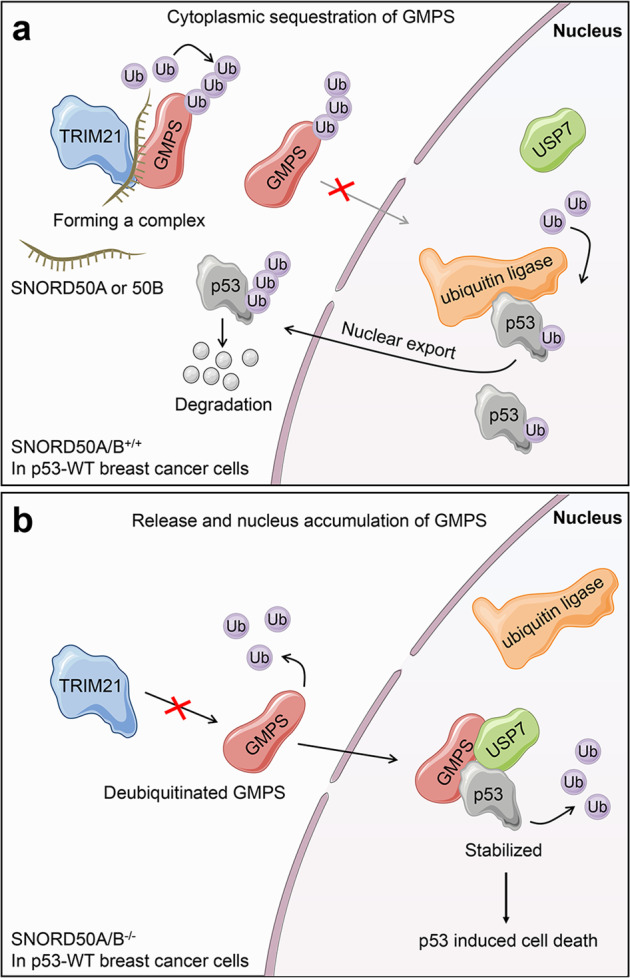
a In general, in p53wt breast cancer cells, SNORD50A/B promotes the interaction between TRIM21 and GMPS by directly binding them. As a result, GMPS is ubiquitinated by TRIM21 and sequestered in the cytoplasm, thereby leading to p53 ubiquitination and degradation. b In SNORD50A/B-deleted p53wt breast cancer cells, GMPS can be released into the nucleus, where GMPS can recruit USP7 and form a complex with p53, thereby decreasing p53 ubiquitination and increasing its protein stability.
