Table 1.
Chronological list of the development of N-F fluorinating agents.
| year | structure | “F” source | yield | references |
| 1964 | 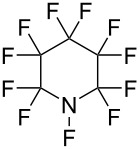 |
HF | 7.5–13% | [16–22] |
| 1981 | 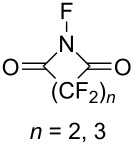 |
XeF2 | 50–65% | [23] |
| 1983 | 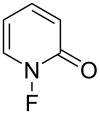 |
5% F2/N2 | 63% | [24–25] |
| 1984 |
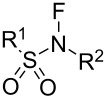 R1 = p-tolyl, n-butyl; R2 = methyl, tert-butyl, exo/endo-2-norbornyl, cyclohexyl, neopentyl |
1–5% F2/N2 | 11–71% | [26–27] |
| 1986– 1991 |
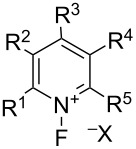 X = OTf, BF4, PF6, AsF6, SbF6, ONf, OMs, etc. R1, R2, R3, R4, R5 = H, Me, Cl, F, COOMe, OMe, CH2OMe, CH2OAc, CF3, CN, NO2, OMenth, etc.; (total 62 examples) |
10% F2/N2 | 15–96% | [28–34,36–41] |
| 1986 | 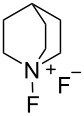 |
100% F2 | 86% | [43–44] |
| 1987 |
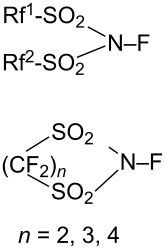 Rf1,2 = CF3, C4F9, C6F13 |
100% F2 | 61–96% | [45–49] |
| 1988 | 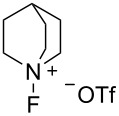 |
100% F2 | 80–88% | [50–51] |
| 1988 |
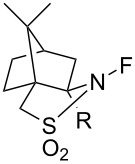 R = H, CH3 |
10% F2/N2 | 75–80% | [52] |
| 1989 | 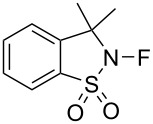 |
10% F2/N2 | 74% | [53] |
| 1990 | 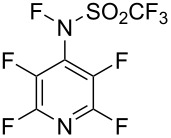 |
100% F2 | 89% | [54] |
| 1990 | 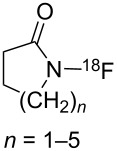 |
0.05% 18F2/Ne | 33–79% | [55–56] |
| 1991 | 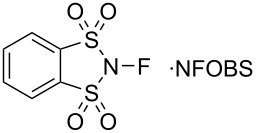 |
10% F2/N2 | >90% | [57–58] |
| 1991 | 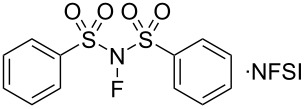 |
10% F2/N2 | 70% | [59] |
| 1991 |
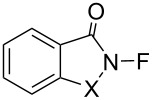 X = SO2, CO |
Cs+-OSO2OF | 48–69% | [60] |
| 1992 |
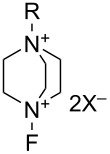 X = OTf, BF4 R = CH3, CH2Cl, CH2CF3 SelectfluorTM (R = CH2Cl, X = BF4) |
10% F2/N2 | 87–95% | [42,61–74] |
| 1993 | 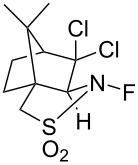 |
10% F2/N2 | 68% | [75] |
| 1995 |
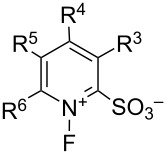 R3, R4, R5, R6 = H, CH3, CF3, Cl, C2H5, tert-butyl |
10% F2/N2 | 65–95% | [76] |
| 1995 |
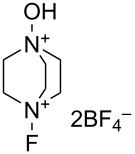 NFTh/AccufluorTM |
10% F2/N2 | 75% | [78–80] |
| 1995 | 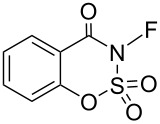 |
5% F2/N2 | 83% | [81] |
| 1996 | 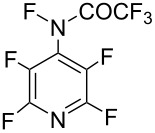 |
100% F2 | 75% | [82] |
| 1996 |
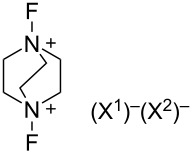 X1, X2 = OTf, BF4, HSO4, SbF6, PF6, etc. |
10% F2/N2 | 56–87% | [83] |
| 1997 |
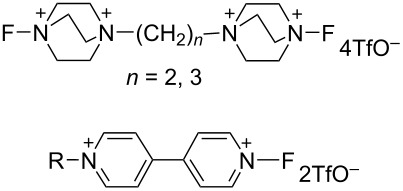 R = BF3, F, CH3 |
10% F2/N2 | 62–82% | [85] |
| 1997 |
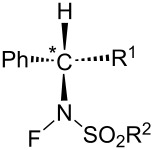 R1 = CH3, CH2OCOCH3 R2 = CH3, p-tolyl |
FClO3 | 13–52% | [92] |
| 1998 |
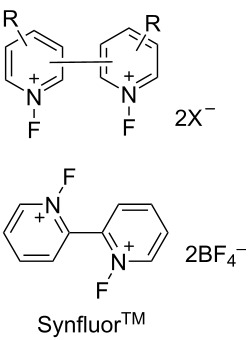 X = OTf, BF4, PF6, SbF6 bipyridyl = 2,2', 2,4', 3,3', 4,4'; R = H, CH3, Cl, CF3, Ph, COOCH3 |
10-20% F2/N2 | 64–97% | [86] |
| 1998 | 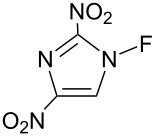 |
5% F2/N2 | – | [87–88] |
| 1999 |
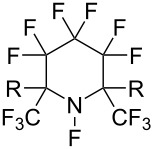 R = CH3, CF3 |
10% F2/N2 | 65–82% | [89] |
| 1999 | 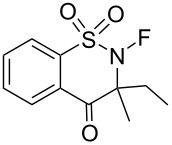 |
FClO3 | 71% | [90] |
| 1999 |
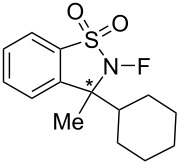 (R)-(+) (S)-(–) |
15% F2/He | 51–65% | [91] |
| 2000 |
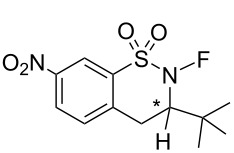 (R), (S) |
FClO3 | 66–83% | [93] |
| 2000 |
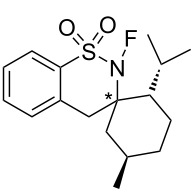 (S,R,R), (S,S,R) |
FClO3 | 44–81% | [94] |
| 2000 | 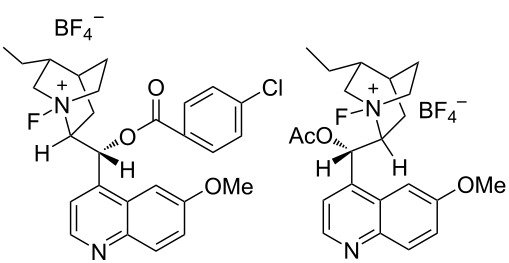 |
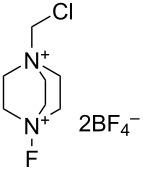 SelectfluorTM |
prepared in situ | [95–96] |
| 2000 |
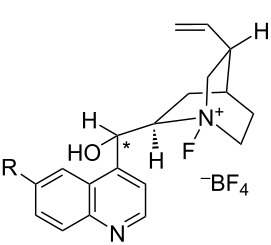 R = H, OCH3 |
SelectfluorTM | 84% | [97–98] |
| 2003 |
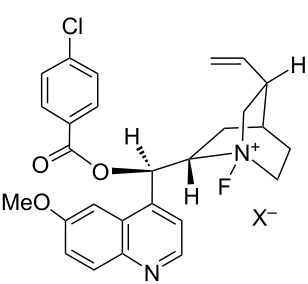 X = BF4, OTf, N(SO2Ph)2 |
Selectfluor (F-TEDA-BF4) F-TEDA-OTf NFTh NFSI N-F-diCl-pyridinium BF4 |
100% | [99] |
| 2003 | 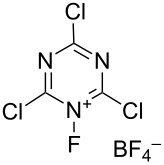 |
100% F2 | 95% | [100] |
| 2011 |
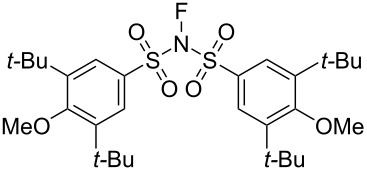 NFBSI |
10% F2/N2 | 57% | [102] |
| 2012 |
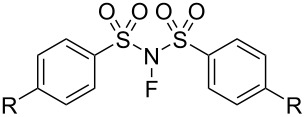 R = F, Cl, Br, CF3, OCF3, t-Bu, Me, OCH3 |
10% F2/N2 | 20.9–89.4% | [103–105] |
| 2013 |
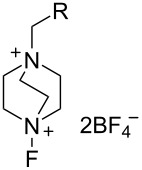 R = 3,5-(CF3)2C6H3, C6F5 |
XeF2 | 29–51% | [106] |
| 2013 |
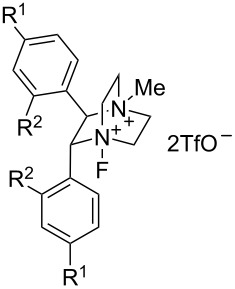 R1 = H, CF3; R2 = H, CH3 |
10% F2/N2 or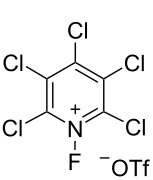
|
>95% | [107] |
| 2013 |
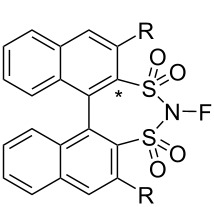 (R), R = H; (S), R = 3,5-(CF3)2C6H3 |
0.2% F2/N2 | 27–67% | [108] |
| 2016 |
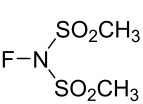 Me-NFSI |
10% F2/N2 | 76% | [111] |
| 2016 | 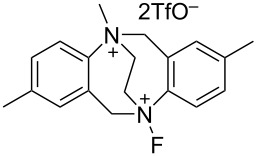 |
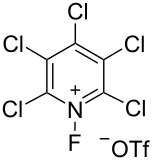 |
>95% conversion | [113] |
| 2018 | 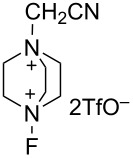 |
20% F2/N2 | 50% | [114] |
| 2018 |
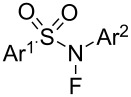 Ar1 = C6H5, 4-Cl-C6H4, 2,4,6-triMe-C6H2, etc.; Ar2 = 4-CF3-C6H4, 3-CF3-C6H4, 3,5-diCF3C6H3 |
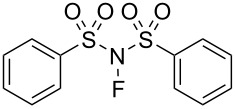 NFSI |
20–93% | [115] |
