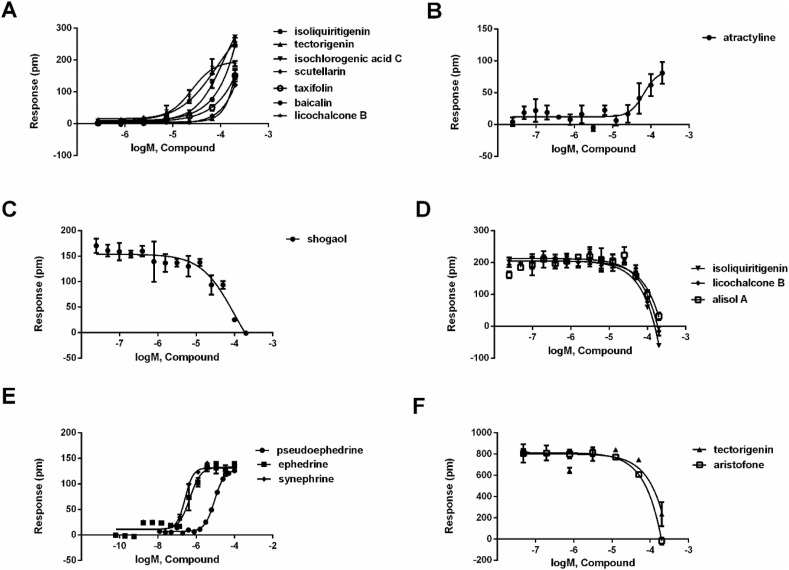
Fig. 3.
Characterization of potential compounds on six targets. (A) The DMR amplitudes of licochalcone B, tectorigenin, taxifolin, isochlorogenic acid C, isoliquiritigenin, baicalin and scutellarin as a function of their concentrations in HT-29 cells. (B) The DMR amplitudes of atractyline as a function of its concentrations in CHO-CB2 cells. (C) The DMR amplitudes of histamine after A431 cells were pretreated with different concentrations of shogaol. (D) The DMR amplitudes of bradykinin after A549 cells were pretreated with different concentrations of licochalcone B, alisol A and isoliquiritigenin, respectively. (E) The DMR amplitudes of ephedrine, pseudoephedrine and synephrine as a function of their concentrations in A431 cells. (F) The DMR amplitudes of acetylcholine after HEK293-M3 cells were pretreated with different concentrations of tectorigenin and aristofone, respectively. All data represent mean ± s.d. from two independent measurements, each in triplicate (n = 6).