Scheme 2.
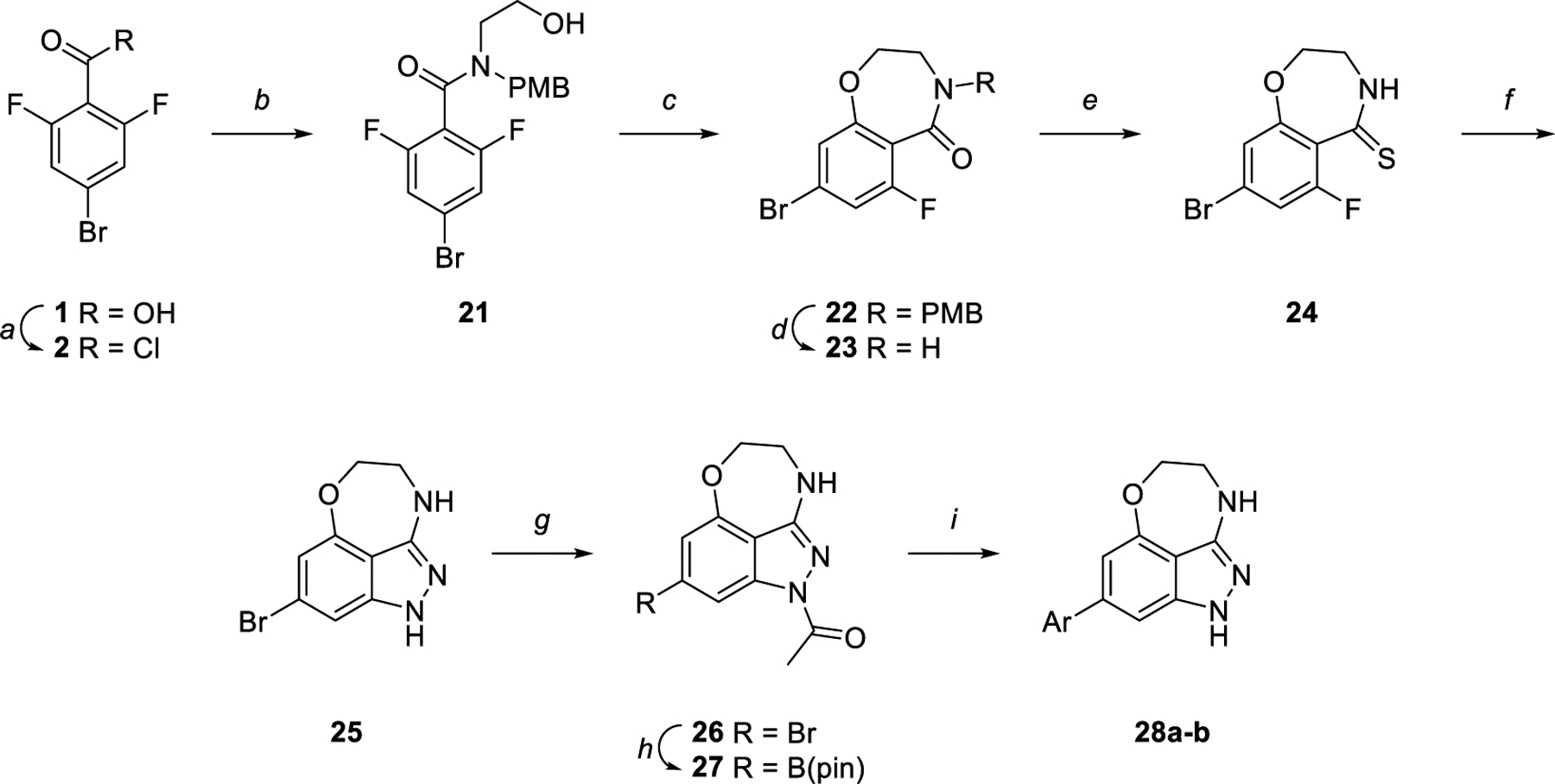
Synthesis of oxazepinoindazole core. Reagents and reaction conditions: a) SOCl2, 75 °C, 4 h (quant.). b) 2-((4-Methoxy-benzyl)amino)ethan-1-ol or ethanolamine, TEA, DCM, 0 °C to rt, 12 h (54%). c) NaH, DMF, rt, 12 h (96%). d) CAN, 3:1 acetonitrile:water, rt, 3 h (36%). e) Lawesson’s reagent, toluene, 100 °C, 12 h (56%). f) Hydrazine, dioxane, 85 °C, 3 h (95%). g) Acetic anhydride, 100 °C, 3 h (60%). h) B2(pin)2, KOAc, PdCl2(dppf)·CH2Cl2, dioxane, 145 °C, μw, 1 h (79%). i) Aryl halide, K2CO3, Pd(PPh3)4, 3:1 dioxane:water, 100 °C, 4 h (12–34%).
