Scheme 3.
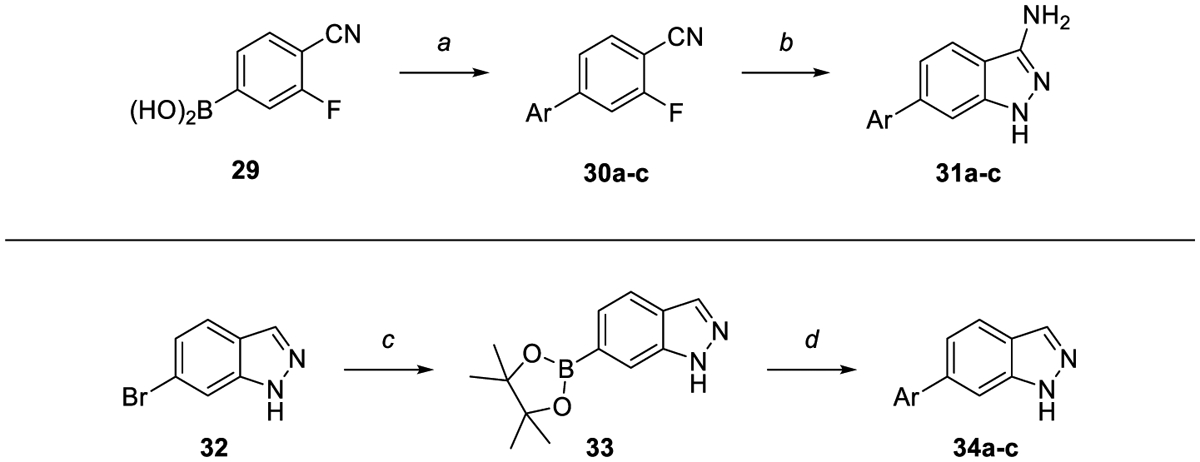
Synthesis of indazoles and aminoindazoles. Reagents and reaction conditions: a) Aryl halide, NaHCO3 (sat. aq.), Pd(PPh3)4, dioxane, 95 C, 3 h (43–97%). b) Hydrazine, EtOH, 95 °C, 12 h (71–87%). c) B2(pin)2, KOAc, PdCl2(dppf)·CH2Cl2, dioxane, 145 °C, μw, 30 min – 1.5 h. d) Aryl halide, K2CO3, PdCl2(dppf)·CH2Cl2, 3:1 dioxane:water, 150 °C, μw 30 min, (6–26%). Where no yield is reported, crude material was progressed without further purification.
