FIGURE 1.
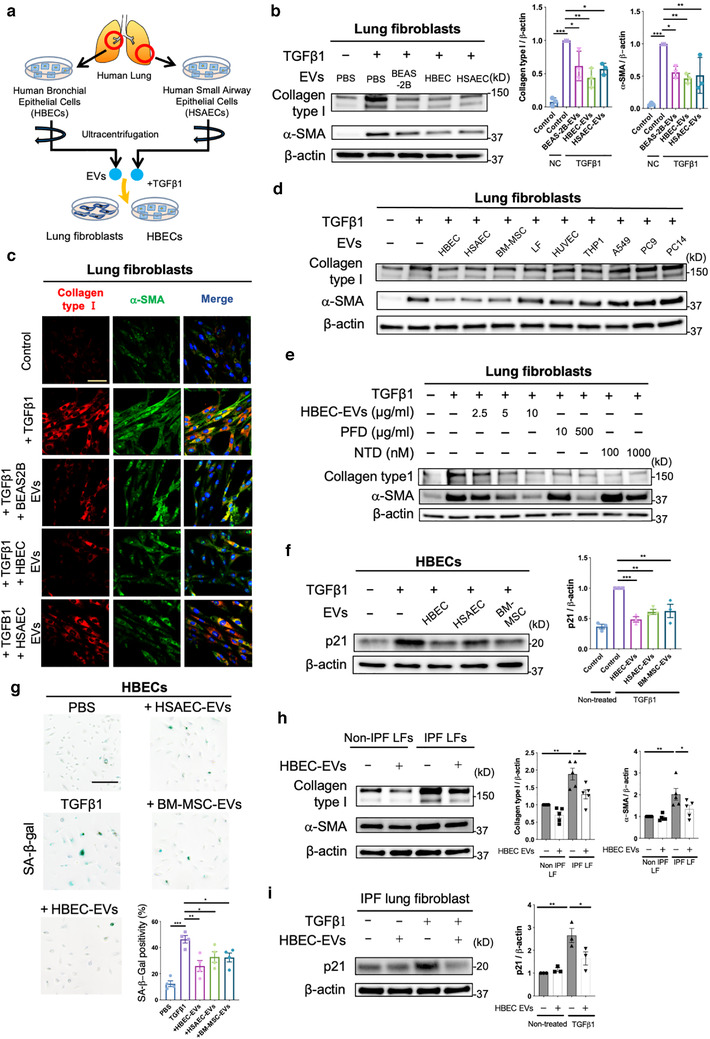
Lung epithelial cell‐derived EVs attenuate TGF‐β‐induced myofibroblast differentiation and lung epithelial cell senescence. a Schematic protocol for the administration of airway epithelial cell‐derived EVs, including human bronchial epithelial cell (HBEC) EVs and human small airway epithelial cell (HSAEC) EVs, to lung fibroblasts (LFs) or HBECs. b Representative immunoblot and quantitative analysis showing the amount of type I collagen, α‐smooth muscle actin (SMA), and β‐actin in LFs treated for 24 h with PBS, BEAS‐2B EVs (10 μg/ml), HBEC EVs (10 μg/ml) or HSAEC EVs (10 μg/ml) in the presence or absence of TGF‐β1 (2 ng/ml). Protein samples were collected from treated cells 72 h after initiation of stimulation. *P < 0.05, ** P < 0.005, *** P < 0.001. c Representative images of anti‐α‐SMA, anti‐type I collagen and Hoechst 33342 staining in LFs incubated for 24 h with PBS, BEAS‐2B EVs (10 μg/ml), HBEC EVs (10 μg/ml) or HSAEC EVs (10 μg/ml) in the presence or absence of TGF‐β1 (2 ng/ml). Staining was performed 72 h after beginning stimulation. Bar: 20 μm. d Representative immunoblot showing the amount of type I collagen, α‐SMA, and β‐actin in LFs treated for 24 h with PBS, HBEC EVs, HSAEC EVs, BM‐MSC EVs, LF EVs, HUVEC EVs, THP1 EVs, A549 EVs, PC9 EVs, or PC14 EVs in the presence or absence of TGF‐β1 (2 ng/ml). EVs were added to the medium at a concentration of 2×109 particles per ml. Protein samples were collected after 72 h of stimulation. e Representative immunoblot showing the amount of type I collagen, α‐SMA, and β‐actin in LFs treated for 24 h with PFD (10 or 500 μg/ml) or nintedanib (NTD) (100 or 1000 nM) in the presence or absence of TGF‐β1 (2 ng/ml). Protein samples were collected 72 h after stimulation. f Representative immunoblot showing the amount of p21 and β‐actin in HBECs treated for 48 h with PBS, HBEC EVs, HSAEC EVs and BM‐MSC EVs in the presence or absence of TGF‐β1 (2 ng/ml). EVs were added to the medium at a concentration of 2×109 particles per ml. Protein samples were collected 96 h after beginning stimulation. ** P < 0.005, *** P < 0.001. g Representative images of SA‐β‐gal staining in HBECs. HBECs were treated for 48 h with PBS, HBEC EVs, HSAEC EVs and BM‐MSC EVs in the presence or absence of TGF‐β1 (2 ng/ml). EVs were added to the medium at a concentration of 2×109 particles per ml. Staining was performed 96 h after the start of treatment. *P < 0.05, **P < 0.005, ***P < 0.001. Scale bar, 200 μm. h Representative immunoblot showing the amount of type I collagen, α‐ SMA, and β‐actin in IPF LFs treated for 48 h with PBS or HBEC EVs (10 μg/ml). *P < 0.05, **P < 0.005. i Representative immunoblot showing the amount of p21 and β‐actin in IPF LFs treated for 48 h with PBS or HBEC EVs (10 μg/ml) in the presence or absence of TGF‐β1 (2 ng/ml). Protein samples were collected 96 h after beginning stimulation. *P < 0.05, **P < 0.005
