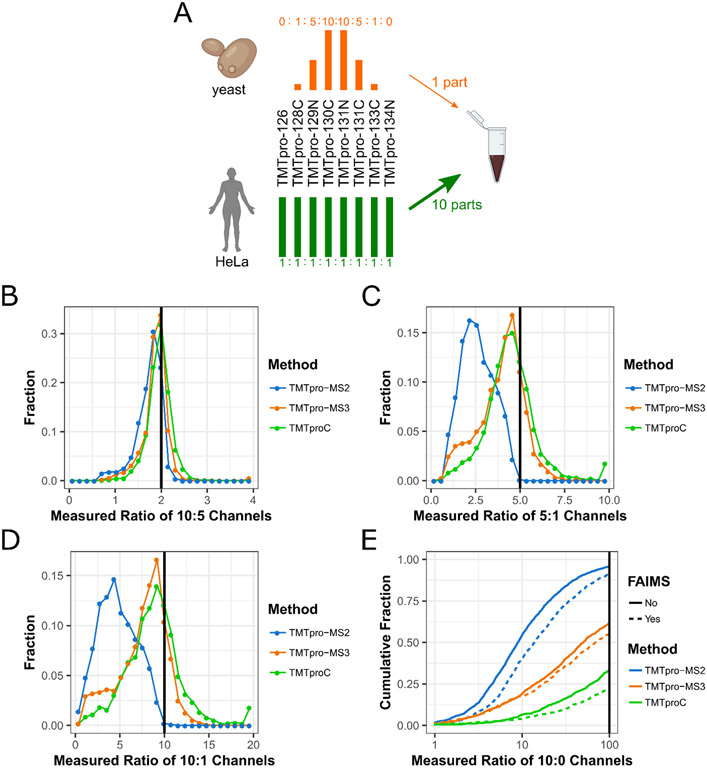
Figure 2. Evaluating ratio distortion for TMTproC and alternative multiplexed quantification strategies.
A) Yeast lysate labelled with TMTpro in ratios of 0:1:5:10:10:5:1:0 was mixed with HeLa lysate labelled with TMTpro in ratios of 1:1:1:1:1:1:1:1 at a mixing ratio of 1 (yeast):10 (HeLa). B-D) Histograms showing the measured ratios of the 10:5 (C), 5:1 (D), and 10:1 (E) channels for yeast peptides with various quantification strategies. Peptides with a sum S:N of less than 200 were removed from this analysis. Measured ratios outside the histogram were set to the closest ratio shown. MS2 quantification of the 10:1 and 5:1 channels is plagued by interference, which MS3 and TMTproC reduce. TMTproC outperforms TMTpro-MS3, since fewer peptides are distorted (shoulders on the left side of the 5:1 and 10:1 histogram). E) The cumulative distribution function of measured ratios for each peptide of the two 10:0 channel pairs was calculated using three different quantification methods, each with and without ion-mobility prefractionation (FAIMS). The measured ratio of the 10:0 channels is plotted against the summed fraction of peptides showing a ratio less than or equal to that ratio. In absence of interference, the ratio would be infinite, but with interference, the ratio is reported as smaller. Therefore, the lower the cumulative fraction of peptides at the high 100 ratio cut-off, the better the method deals with interfering peptides. Peptides for which the sum of the quantifiable ions signal to noise ratio (S:N) was less than 500 were removed for all methods. Other signal to noise cutoffs can be found in Sup Fig. S5. Measured ratios greater than 100 were set to 100. TMTpro-MS3 reduces interference compared to TMTpro-MS2. TMTproC reduces interference even further compared to TMTpro-MS3.