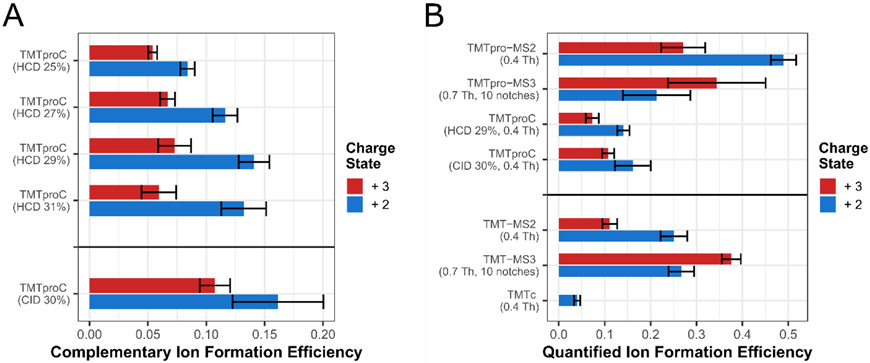
Figure 3. Optimization of TMTproC ion formation and comparison with ion formation efficiencies in alternative multiplexed proteomics methods.
A) Evaluation of various fragmentation methods for TMTproC ion formation. HeLa peptides were labelled with TMTpro0, subjected to various fragmentation schemes and analyzed five times across several weeks of normal instrument use. For each run, the median proportion of MS1 ion flux from the precursor peak that was converted into complementary ions was calculated. Shown are the mean of five runs along with error bars of one standard deviation. On average, CID fragmentation produced 15% more signal in the complementary ion cluster than HCD fragmentation, but this difference was not significant (Student’s t-test, p-value=0.31). B) Comparison of TMTproC ion efficiencies with alternative methods. HeLa peptides labeled with TMTpro0 or TMT0 were subjected to various quantification methods. For each, we calculate the median fraction of precursor ion flux that is converted into the relevant ion used for quantification and then calculated the mean across multiple runs (five for TMTproC methods, four for all others) along with error bars of one standard deviation. TMTproC improves complementary ion formation efficiency to nearly the same level as MS3 reporter ion-based quantification methods (Student’s t-test, p-value=0.27).