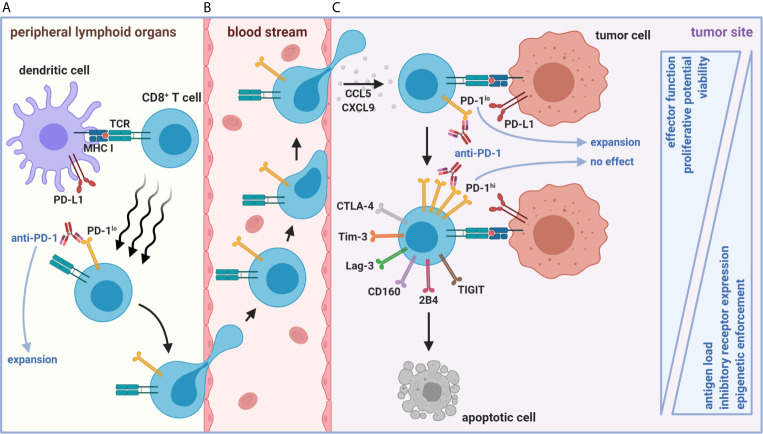
Figure 2.
Spatiotemporal organization of early versus late stages of tumor-mediated CD8+ T cell dysfunction. (A) Naïve CD8+ T cell priming against tumor antigen in peripheral LNs (or intratumoral TLS, not depicted) results in the formation of a stem-like PD-1loCD8+ T cell population with self-renewing properties. (B) This population represents an active reservoir of cells that can give rise to effector-like PD-1loCD8+ Tex after chemokine-mediated trafficking to and positioning within the TME via CCL5 and CXCL9. (C) However, persistent antigen load in the TME eventually forces continued differentiation of these cells into terminally dysfunctional PD-1hiCD8+ Tex. The PD-1hi state is accompanied by heightened co-inhibitory receptor expression (including Tim-3, Lag-3, CD160, 2B4, TIGIT, and CTLA-4) and progressive loss of effector functions. Once CD8+ Tex enter a PD-1hi state, epigenetic enforcement prevents de-differentiation back to functional stem-like and effector-like PD-1lo states. Anti-tumoral responses facilitated by ICB (e.g., anti-PD-1) arise from expansion from only lymphoid or intratumoral PD-1loCD8+ Tex subsets. The functionally inferior, ICB-resistant PD-1hiCD8+ Tex fate ultimately culminates in apoptosis.