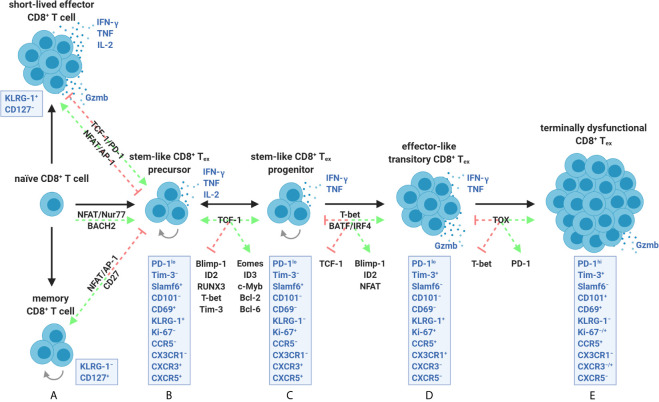
Figure 4.
The CD8+ T cell exhaustion lineage is comprised of a continuum of transcriptionally and epigenetically controlled states. (A) Activation of naïve CD8+ T cells for SLEC and MPEC/memory differentiation is optimally driven by transcription factors such as NFAT/AP-1 and sufficient co-stimulation (e.g., CD27). Early development of stem-like CD8+ Tex precursors instead involves partnerless NFAT, lack of co-stimulation/help, constant Nur77 activity, and/or strict dependence on BACH2. These events appear to be stabilized by TCF-1 activity and PD-1 dampening of chronic TCR ligation. (B, C) TCF-1 further supports stemness (ability to survive, self-renew, and proliferate) by promoting Eomes, ID3, c-Myb, Bcl-2, and Bcl-6 expression while antagonizing effector-associated transcription factors including Blimp-1, ID2, RUNX3, and T-bet. Stem-like CD8+ Tex precursors (B) and progenitors (C) are collectively marked by a PD-1loTim-3−Slamf6+ surface profile and varied expression of CXCR3 and CXCR5 in specific tumor settings. Although equally stabilized by TCF-1, precursors can be distinguished from progenitors as being quiescent, LN-resident, less reliant on antigen, and having a CD69+KLRG-1+Ki-67− profile. (C, D) TCF-1 down-regulation coupled with ongoing exposure to persistent antigen drives constant BATF and IRF4 signaling (which positively feedback on partnerless NFAT activity) and T-bet expression. T-bet additionally overrides a TCF-1 memory-like program by supporting Blimp-1 and ID2 activity leading to an effector-like transitory PD-1loTim-3+ state marked by initiation of granzyme B production. (D, E) Continued NFAT activity ultimately leads to TOX upregulation within this subpopulation, which epigenetically enforces terminal exhaustion, inhibits T-bet-mediated effector programming, and promotes heightened PD-1 expression. Transitory cells (D) are discriminated from terminally dysfunctional cells (E) by a PD-1loTim-3+CD101−KLRG-1+CX3CR1+ surface phenotype, remnant IFN-γ and TNF production, and having high proliferative potential. Terminally dysfunctional PD-1hiTim-3+CD8+ Tex co-express multiple co-inhibitory receptors (not depicted), cannot proliferate, have diminished polyfunctionality, but retain granzyme-based cytolytic potential. PD-1lo precursors, progenitors, and transitory CD8+ Tex subpopulations are amenable to ICB (B–D), whereas terminally dysfunctional PD-1hiCD8+ Tex are not (E). Precursors and progenitors may interconvert, whereas differentiation into transitory and terminally dysfunctional subsets is unidirectional.