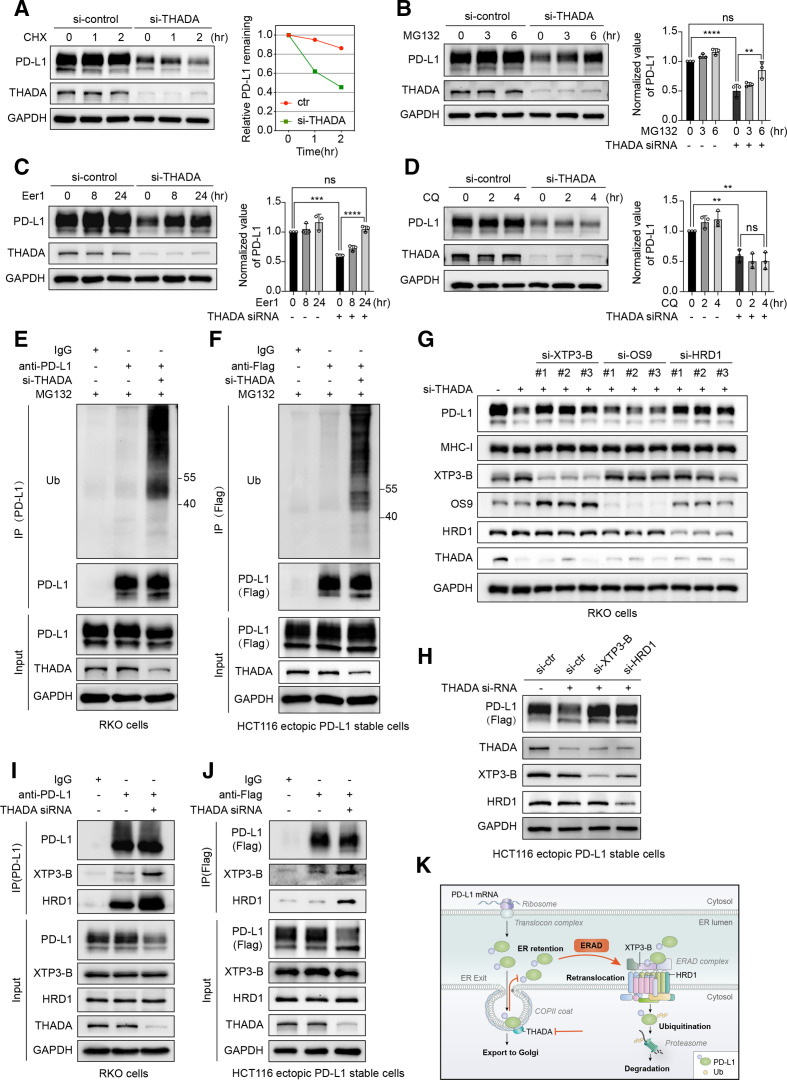
Figure 4.
Thyroid adenoma associated gene (THADA) depletion induced endoplasmic reticulum (ER)-associated degradation of programmed death-ligand 1 (PD-L1). (A) Left, RKO cells transfected with THADA small interfering RNAs (siRNAs) and co-incubated with cycloheximide (CHX) (50 µg/mL) for denoted time points, and subjected to western blot analysis with indicated antibodies. Right, quantification of gray value of remained PD-L1. The experiment was repeated three times independently with similar results. Left, western blot analysis showing the effect of THADA depletion on PD-L1 expression in the absence or presence of proteasomal inhibitor MG-132 (10 µM) (B), endoplasmic reticulum-associated degradation (ERAD) inhibitor eeyarestatin I (Eer I) (10 µM) (C), lysosomal inhibitor chloroquine (CQ) (25 µM) (D) in LoVo cells. Right, values are means±SD from three independent experiments. Statistical differences were determined by analysis of variance (ANOVA) post hoc test (Tukey). **P<0.002; ***p<0.0002; ****p<0.0001; ns, no significance. Western blot analysis showing the ubiquitination of endogenous PD-L1 in RKO cells (E) and exogenous PD-L1 in HCT116 ectopic PD-L1 stable cells (F). Western blot analysis evaluating the expression of endogenous PD-L1 (G) and exogenous PD-L1 (H) in the indicated cells transfected with XTP3-B, HRD1, OS-9 and THADA siRNAs as denoted. Co-immunoprecipitation (Co-IP) assays revealing the interactions between XTP3-B/HRD1 and endogenous PD-L1 (I)/exogenous PD-L1 (J) in the indicated cells. The experiments were repeated three times independently with similar results, respectively. (K) Schematic displaying that THADA knockdown blocks PD-L1 trafficking and detains PD-L1 in the ER, thereby causing PD-L1 to be recognized, targeted and retrotranslocated by ERAD complex containing E3 ligase HRD1 and lectin-like protein XTP3-B, and ultimately degraded by ubiquitin-proteasome pathway in the cytoplasm.