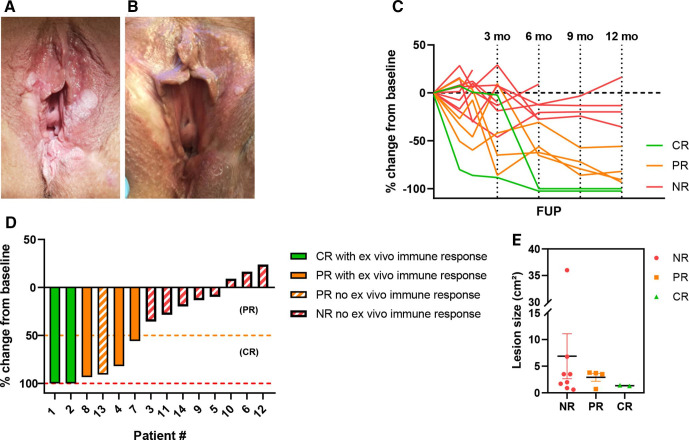
Figure 2.
clinical response data of cohort 1 and 2. (A) Usual vulvar intraepithelial neoplasia (uVIN) lesions visible at screening visit. (B) Partial response of uVIN lesions visible at follow-up +12 months after vaccination with human papillomavirus-16 E6/E7 tattoo vaccination. (C) Overview of uVIN lesion size changes (as percentage change compared with baseline) during follow-up. (D) Waterfall plot showing percentage change of uVIN lesion at last follow-up compared with baseline lesion size (=lesion size at screening). (E) Lesion size before therapy per response category. Complete responders are depicted in green, partial responders in orange and non-responders in red. CR, complete response; NR, non-responder; PR, partial response.