Extended Data Fig. 4|. Characterization of BAHCC1BAH binding to H3K27me3 through their co-crystal structure and structure-based mutagenesis.
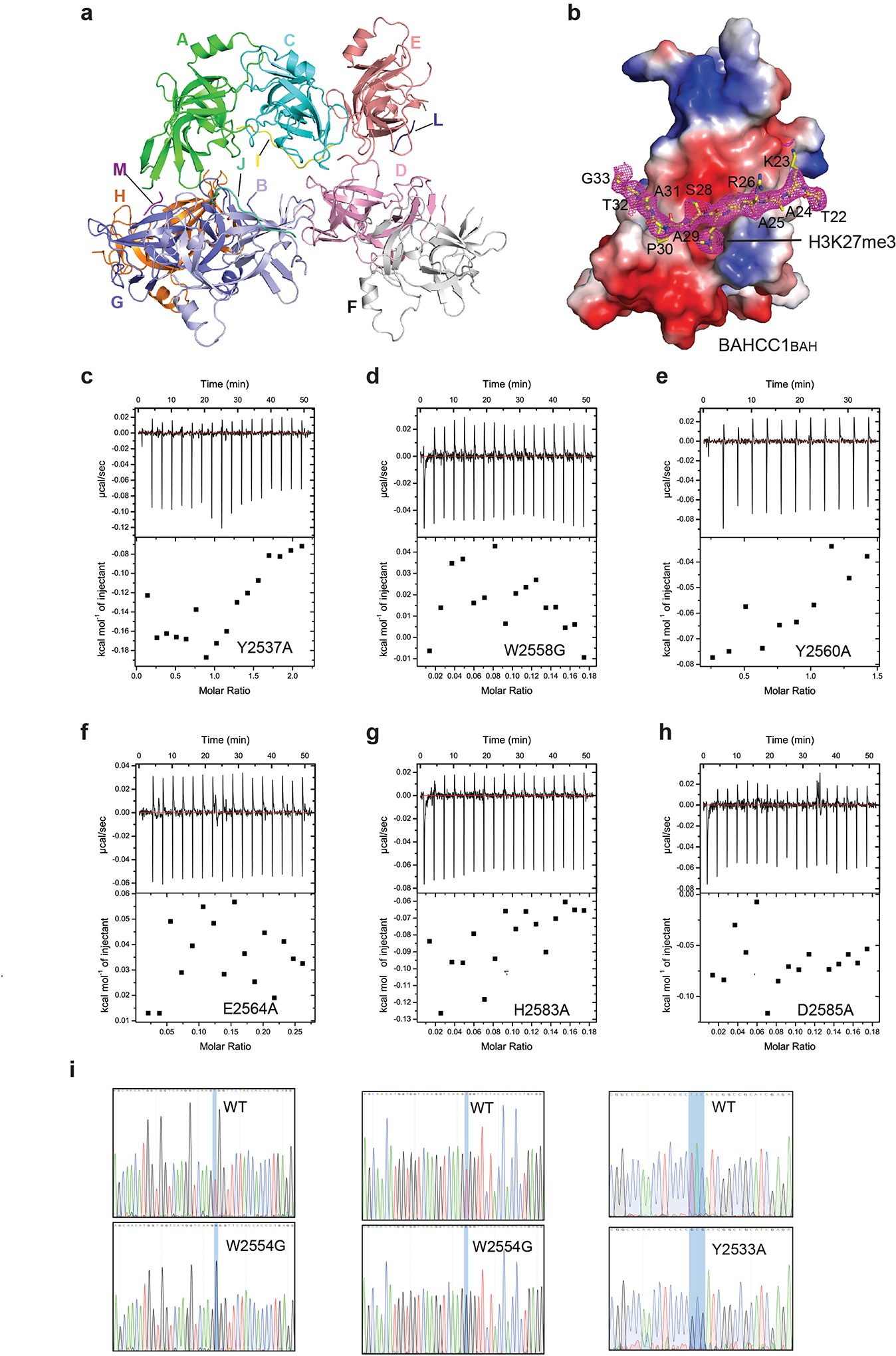
a, Crystal structures of the eight color-coded murine BAHCC1BAH molecules in one asymmetric unit, with chain ID labeled. Four of the BAHCC1BAH molecules (chain B, C, E and G) are complexed with H3K27me3 peptides (chain J, I, L and M, respectively.). The BAHCC1BAH:H3K27me3 complex with the best model-to-map fit (Chain C and I) was selected for structural analysis.
b, Electrostatic surface view of murine BAHCC1BAH bound to the H3K27me3 peptide (yellow sticks). The Fo-Fc omit map for the H3K27me3 peptide, contoured at 1.5 sigma level, was shown as mesh in magenta.
c-h, ITC binding curves of the H3K27me3 peptide with murine BAHCC1BAH recombinant protein carrying an indicated mutation, either Y2537A (c), W2558G (d), Y2560A (e), E2564A (f), H2583A (g) or D2585A (h).
i, CRISPR-cas9-mediated genomic editing to introduce homozygous point mutation of W2554G (left two panels) or Y2533A (right) into the BAHCC1 gene in JURKAT cells. Shown are Sanger sequencing results using cDNA (middle) or genomic DNA (left and right) as template. See also Extended Data Fig. 3g for murine and human BAHCC1 amino acid numerations.
