Fig. 2. BAHCC1BAH specifically ‘reads’ H3K27me3.
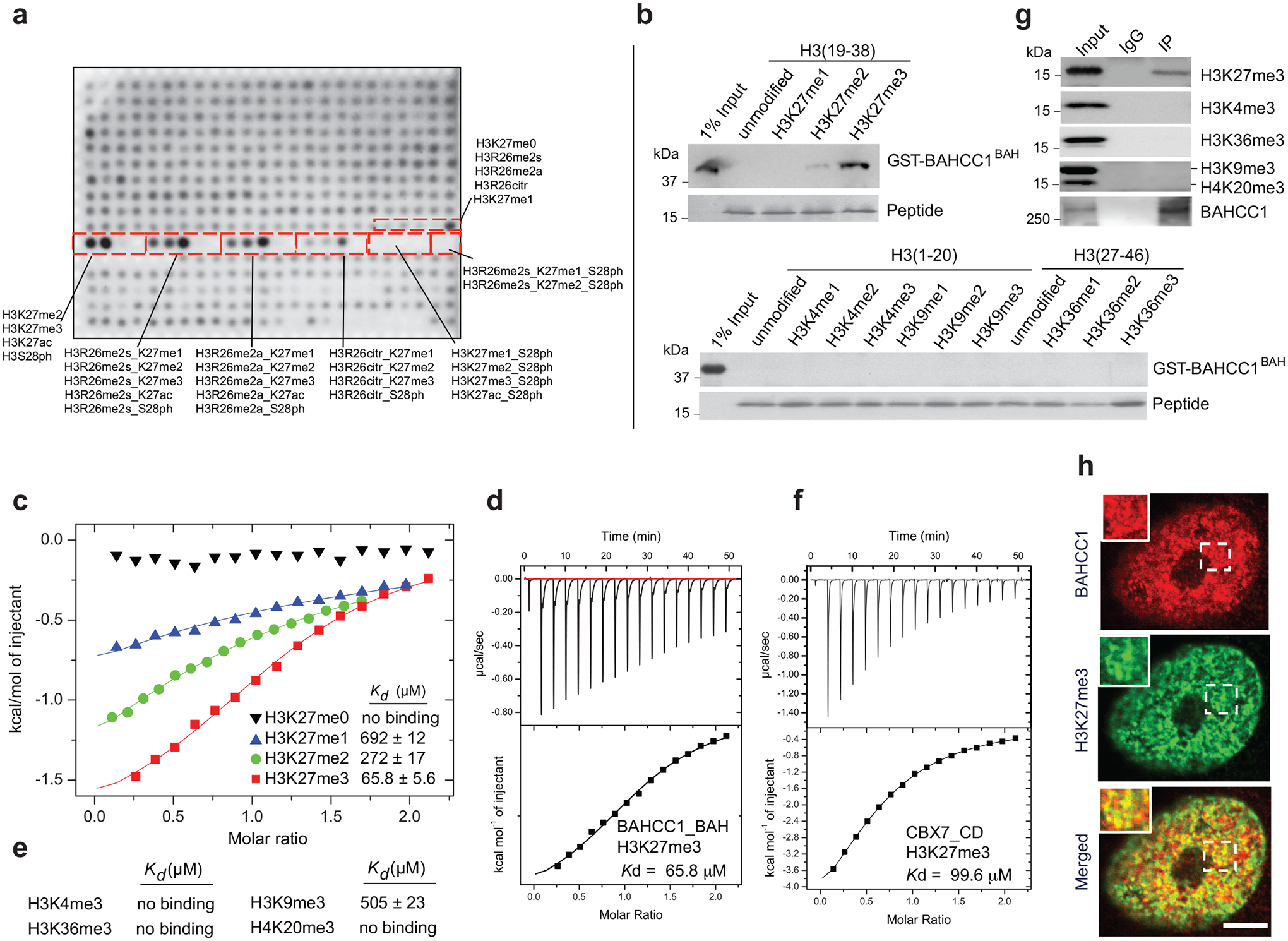
a, Histone peptide array probed with GST-BAHCC1BAH protein, followed by visualization with anti-GST antibody. Red boxes highlight peptides carrying H3K27 methylation, either singly or in combination with adjacent residue modification, with the corresponding peptide identification annotated aside.
b, Pulldown using GST-BAHCC1BAH and biotinylated histone peptide carrying H3K27 methylation (top) or the other indicated methylation (bottom).
c, ITC measuring affinity of binding between BAHCC1BAH and peptide with H3K27 methylation.
d, ITC binding curve of BAHCC1BAH recombinant protein with the H3K27me3 peptide.
e, Summary of ITC measurements revealing affinities of binding between BAHCC1BAH and the indicated histone lysine trimethylation.
f, ITC binding curve of CBX7CD protein with the H3K27me3 peptide.
g, CoIP for interaction between endogenous BAHCC1 and the indicated histone methylation in JURKAT cells.
h, Representative images of confocal immunofluorescence microscopy showing that BAHCC1 (red) colocalizes with H3K27me3 (green) in HeLa cells. Scale bar, 5 μm.
