ABSTRACT
Most textbook definitions recognize only animals as having nervous systems. However, for the past couple decades, botanists have been meticulously studying long-distance signaling systems in plants, and some researchers have stated that plants have a simple nervous system. Thus, an academic conflict has emerged between those who defend and those who deny the existence of a nervous system in plants. This article analyses that debate, and we propose an alternative to answering yes or no: broadening the definition of a nervous system to include plants. We claim that a definition broader than the current one, which is based only on a phylogenetic viewpoint, would be helpful in obtaining a deeper understanding of how evolution has driven the features of signal generation, transmission and processing in multicellular beings. Also, we propose two possible definitions and exemplify how broader a definition allows for new viewpoints on the evolution of plants, animals and the nervous system.
KEYWORDS: Plants, animals, nervous system, evolutionary convergence
1. Introduction
Under the entry for “nervous system”, Encyclopædia Britannica states that [i]n animals, in addition to chemical regulation via the endocrine system (which plants have), there is another integrative system called the nervous system.1 Most dictionaries and textbooks maintain that only animals have nervous systems. Although plants do not have a nervous system according to this phylogenetic definition, a growing body of botany research from the past 25 years shows that many plants transmit electrical signals to and from different parts of their bodies to respond to environmental stimuli.2 Several scientists from different fields have spoken about plant neurobiology and the nervous systems of plants,3–5 but this new viewpoint is not free of controversy and has been criticized by others.6,7 One could consider this controversy to be an ontological debate about whether some entities belong to one category or another, not affecting physiological plant and animal research, but this debate is not harmless to scientific knowledge – it is significant in evolutionary biology because a phylogenetic definition does not allow for considering processes of convergence evolution, which is necessary when discussing the evolution of living beings.8–10
In this article, after reviewing the scientific literature on electrical signaling in plants and discussing the criticisms of plant neurobiology from an evolutionary point of view, we propose that broadening the definition of a nervous system will provide a greater understanding of the evolution of how plants and animals generate, transmit and process signals. To highlight the advantages of a broader definition, we use a definition that allows us to discuss the evolutionary directions of different nervous system features in plants and animals. In this paper, we do not address the existence of consciousness in plants nor take sides on this issue.11–14 We separate the issues of consciousness and the nervous system. Although we conceive of consciousness as a functional state that exists in at least some nervous systems,15,16 consciousness continues to be one of the most unknown phenomena of nature, despite research progress in this area.17–20 Thus, our approach to the debate about nervous systems in plants is only from a physiological perspective, by which we take into account only the generation, transmission and processing of signals
We are aware that formulating a definition for the term “nervous system” is challenging because it needs to be general enough to allow discussing convergent and divergent evolutionary processes but not so general that it becomes meaningless by including any system of signals. Proof of the difficulty of this challenge is the fact that Elsevier’s Encyclopedia of Neuroscience21 does not have an entry for the term “nervous system”. While this task is likely to fail because of this difficulty, we think that the possibility of gaining a better understanding of nature makes the attempt to formulate a definition worthwhile.
This paper is structured as follows: Section 2 reviews the most important literature about electrical signal generation, transmission and processing in plants. Section 3 reviews textbook definitions of nervous systems and the differences between physiological and anatomical definitions of biological systems. We also discuss criticisms of plant neurobiology from an evolutionary viewpoint. Section 4 presents two options to redefine the concept of what a nervous system is. Section 5 shows how broadening the definition of “nervous system” allows for discussing the evolutionary directions of the signaling features of plants and animals. Section 6 presents a summary and conclusions of the importance of broadening the definition in evolutionary biology.
2. Plant behaviors and the mechanisms of electrical signals
Throughout history, people generally thought of plants as passive organisms, disconnected from information in their environment and performing mechanical functioning without communicating between their organs and structural parts. This view, however, began to be questioned during Darwin’s time after research on electrical signals in plants was published. Motivated by conversations with Darwin about the Venus flytrap,22 John Burdon-Sanderson conducted the first experiment that registered an action potential in a plant.23
Later, Jagadis Chandra Bose performed experiments that demonstrated the electrical nature of signals generated in different plants by different stimuli (e.g., nondestructive electrical shocks, wounds, chemical agents).24–26 His findings were astonishing because at that time is was thought that plants use hydromechanical mechanisms to transmit signals, unlike animals, which use electrical impulses. His studies also showed that electrical signals exist in both sensitive and nonsensitive plants. Despite the topicality of the debate we address in this paper, the idea that plants have a nervous system goes back to Bose. He wrote:
“The results of the investigation which I have carried out for the last quarter of a century establish the generalization that the physiological mechanism of the plant is identical with that of the animal.” [26, p. ix]
Since then, many more studies have confirmed that plants respond quickly by generating, transmitting, and processing electrical signals. In the following text, we briefly review some of the most important research and discoveries about plant behavior and signaling that have been reversing our initial view of plants.
Within the plant kingdom, some sensitive vascular plants, such as the Venus flytrap (Dionaea muscipula), have been studied in depth.27,28 The Venus flytrap uses leaves to capture insects, and it has to make two predictions to be successful.29 First, it must decide whether what is on the leaf is foodstuff to prevent it from closing on sand or other useless materials. The Venus flytrap distinguishes inanimate objects from prey by registering two mechanical stimuli within 2030 seconds. When this occurs, the leaves jump to a semi-closed state. Second, the plant must decide whether the prey is worth closing for. If the insect is too small and escapes through the gaps, the plant will not register additional signals. If the insect is the appropriate size, it will evoke further mechanical stimuli, and the trap will fully close. Additionally, the Venus flytrap can jump from an open state to a semi-closed state in 0.03 seconds, and from a semi-closed to a fully closed state in 0.03 seconds. Electrical signals play an important role in these high-velocity reactions and leaf movements by propagating waves of action potentials.30,31 The Venus flytrap uses the distinct electrical states of its cells to process information,29 and this mechanism could allow for reacting to a wide range of prey movements.32 Also, it has been observed that the Venus flytrap has several interconnected electrical circuits.28 Despite these interesting features, this plant is not an oddity. Aldrovanda and Utricularia are other carnivorous plants that use action potentials to generate predatory behaviors.33
Mimosa pudica is another widely studied plant with striking behavior.34–36 Mimosa pudica and Venus flytrap show how different evolutionary pressures have caused these plants to possess mechanisms that employ electrical signals. Whereas the Venus flytrap carries out predatory behavior, the Mimosa pudica employs electrical signaling to carry out antipredator behavior.37.38.40 It folds its leaves inward when stimulated, reducing the surface area exposed to potential predators. If it continues to receive stimulation, the petiole drops and causes the potential predator to fall from the leaves. A remarkable fact discovered about the Mimosa pudica is that its antipredatory behavior is condition–dependent, like in animals.34
Although sensitive plants can generate high-velocity action potentials that lead predatory and antipredatory movements, these electrical signals are also used to send alerts about wounds and communicate between different tissues and organs.41–44 Electrical signals also elicit changes in physiological processes in ordinary plants.44–46 Also, it has been observed that circadian rhythms in plants have electrical components [47,39], and a new line of research involves electrical communication between plants. For example, researchers found electrical signal conduction between Aloe vera and tomato plants.48,49
Plants can generate electrical signals for both short and long distances, and the propagation of electrical signals can be active or passive, as they are in animals.36 The three types of long-distance electrical signals are the action potential, variation potential (or slow wave potential), and system potential.2 Action potentials in plants are important for transmitting information,50 and their electrical activity contains information about environmental stimuli.51,52 The details of the ionic currents of action potentials in Viridiplantae began to be uncovered in the early 1960s.53,54 But, in contrast with our deep knowledge of the animal nervous system at the molecular level, what we know about the channels involved in depolarization and repolarization in higher plants remains mostly conjecture.
Despite this problem, research has shown that plants’ action potentials are associated with an increase of and concentrations and a decrease of and in the cytoplasm, and the same concentrations change conversely in the apoplast.2 However, although voltage-sensitive channels55 and voltage-sensitive channels56 have been identified, for most of the proposed molecular mechanisms, there is no experimental proof that they are involved in action potentials. Studies have only recently confirmed that voltage-gated potassium channels responsible for repolarization exist57 and that the proton pump AHA1 is implicated in variation potential generation by controlling the membrane potential.58
Regarding long-distance signal transmission, plants use phloem (parenchyma cells, companion cells and the phloem sieve tubes) and xylem to form a network to transmit electrical signals long-distance within the plant.26,36,45,59,60 In maize plants, experiments have shown that different electrical signals existed in a sieve element of their phloem in response to different kinds of leaf tip stimulation. The recordings showed that action potentials were released when the stimulation involved chilling the plants, yet variation potentials occurred when the stimulation involved wounding the plants by cutting. The molecular mechanism of spreading action potentials is debated. So far, it has not been found that plants have chemical synapses to carry out cell-to-cell communication. One hypothesis was that the electrical field strength might be the element in the jumping transmission,61 but the results of a subsequent research denied that hypothesis.62 Another hypothesis about the spreading is that plasmodesmata, membrane-lined channels,63 play the same role in plants that gap junctions play in the animal nervous system and allow for electrical connection and action potential propagation.45,64,65 Xylem plays an important role in the propagation of variation potentials. A recent study using mutant plants found that xylem is related to the velocity and kinetics of variation potential transmission in Arabidopsis.66 In regard to which types of cells and channels are involved in variation potentials, research has revealed important details in Arabidopsis thaliana. Using a genetic approach, the researchers found that this plant contains two distinct populations of cells necessary for transmitting electrical signals and that these cells do not contact each other directly.67 They also found that phloem sieve tubes and xylem contact cells function together in the variation potential. The research also showed that insects feeding on genetically modified plants (those unable to send electrical signals) gained weight more rapidly than those feeding on wild-type plants, indicating that insects found it more difficult to predate plants with systems for sending electrical signals. This study reveals the existence of an evolutionary pressure, insect feeding, that caused the electrical signaling system of plants to be selected because it makes it difficult for insects to prey upon them.
If signals exist, mechanisms to generate and interpret signals must exist. The generation of signals by mechanoreceptors has been an important research topic.68 In the case of the Venus flytrap, the electrical signals that generate its mechanoreceptor have been studied in depth,30 along with its molecular mechanisms.69 The Mimosa pudica’s mechanoreceptor and the mechanisms of its electrical signals are not yet known.37 In the Arabidopsis thaliana, several key discoveries have been made in the last several years about the channels involved in recognizing herbivory70 and the pathogens71,72 that activate defense signaling processes. Despite these discoveries, the mechanisms responsible for transforming a long-distance electrical signal into a cellular response remains an under-explored issue. Even so, it is interesting that a recent study has provided important evidence of the mechanisms involved in decoding variation potentials into a specific respiratory response in pea seedlings.73 Regarding the mechanisms that interpret signals, plant cells decipher information encoded in calcium oscillations that are induced in cytosolic free calcium,74 and several important discoveries about how the calcium signals are read or decoded have shown that the interplay of channels, transporters and sensors are involved in those processes.75
One important issue to consider in the research of any natural phenomenon is its mathematical description. Basic research on electrical signaling in plants has led to important developments in mathematically understanding electrical activity in plants.76 When analyzing those mathematical models, an important issue emerges: many of the elaborated mathematical models are modifications of the classical Hodgkin-Huxley model.
The similarities do not stop there; some chemical messengers used by animal nervous systems have also been found in plants. Two important discoveries have revealed that plants also use glutamate77 and gamma-aminobutyric acid (GABA) as signaling molecules.78 Glutamate interacts with electrical signaling and with other signaling molecules.79 For example, glutamate plays a role in wound signaling; when it is detected by glutamate receptor-like ion channels, they increase the intracellular calcium ion concentration, which creates a signal that propagates through the plant.80 Also, a recent study in Nitellopsis obtusa has given new evidences that NMDA is an active component in glutamatergic signaling in at least some plants.81 Regarding GABA, there are still open issues about its role in signals within plants,37,82,181 but we know that it appears in plants for defense against herbivores and in response to extreme temperatures, dehydration, salinity, oxygen stress, mechanical damage, acidosis and viral infection.83,84 We also know that GABA inhibits anion passage through the aluminum-activated malate transporter.78
The similarities between the signaling mechanisms of plants and animals raise the question of whether animal anesthetics affect plants in a manner similar to animals, and the results of various research studies show that it does occur.85–88 A recent study even found that animal anesthetics abolish movements in several sensitive plants and action potentials in Venus flytraps.89
But the similarities between plants and animals go beyond the mathematical description of their electrical signals and the molecular mechanisms that generate them. Similarities have even been found between the electrical signal systems of different plants. For example, the response in the touch-perception process in Chara (freshwater environments) and the turgor-regulating response to osmotic shock in Lamprothamnium (salt-tolerant types) are both generated by the same three stages.90 In Arabidopsis, petiole distortion from insects resembles certain aspects of the distal leaf collapse phase that can be seen in damaged Mimosa pudica.66 These discoveries indicate that these signal systems have an evolutionary history.
Although we have discussed many details about plant signaling mechanisms, there are still controversies surrounding this topic. One of these is the proposal that the plant signaling system has properties in common with neuronal synapses.91,92 Some researchers claim that root apex cells secrete vesicles enriched with auxin to exchange information among themselves,93,94 but others question these findings,95,96 generating a dispute about the question.97,98 Probably, in the coming years, the use of new techniques is going to clarify the issue.99
The above paragraphs have reviewed important discoveries about signaling in plants, but we have not yet mentioned the degree of complexity of their behaviors and capacities. These controversial issues go back to the time of Darwin. In fact, one of the oldest and most surprising debates concerns the “root-brain” hypothesis proposed by Charles and Francis Darwin.3 The Darwins proposed that roots behave as lower animals do, with their apex seated at the anterior pole of the plant body where it acts as a brain-like organ. In light of the recent discoveries about the root apex, the Darwins’ proposal has been reconsidered. It has been proposed that the root apex is a brain-like structure in plants100 and that the root apex transition zone receives sensory information from the root cap and instructs the motor responses of cells in the elongation zone.100 The “root-brain” hypothesis has been supported by testing binary decisions made by maize roots in a Y-maze.101 Also, spatiotemporal dynamics of electrical network activity have been registered in the root apex, and researchers have proposed that its function is to integrate internal and external signaling for developmental adaptations in response to changes in the environment.33 The experiments have also shown that the electrical activity of the maize root apex is affected by gravity conditions, as happens in animals’ nervous systems.102
The capacity of plants to learn is also an intriguing topic. Although researchers have found interesting results from studying the leaf-folding habituation of Mimosa pudica,103,104 there are discussions about how to interpret the data. Researchers question whether a process of habituation occurs or whether the results can be explained by motor fatigue.99,104,105 To resolve this discussion, additional experiments that contrast the viewpoints are needed.
Another intriguing topic is the mechanisms parasitic plants use to make decisions. They have the particularity of having to locate a host, and it has been shown that Cuscuta europaea has the ability to choose between hosts depending on host quality.106,107 It seems plausible that Cuscuta europaea uses signals to touch its environment,108 but we still know little about its mechanisms to make decisions.
Despite ongoing debates, the facts reviewed here show that plants possess a system that uses electrical signals to sense stimuli and generate behavior to fit into the environment and that this system has an evolutionary history. Thus, the question arises as to whether this system is a nervous system.
3. The definition of a nervous system
Most dictionaries use phylogenetic definitions to identify nervous systems, such as the following from Collins English Dictionary: the sensory and control apparatus of all multicellular animals above the level of sponges, consisting of a network of nerve cells.109 Other dictionaries opt to define a “nervous system” by directly linking it to the kingdom Animalia or to one of its taxonomic ranks; for example, the Cambridge Advanced Learner’s Dictionary states that an animal’s or person’s nervous system consists of its brain and all the nerves in its body that together make movement and feeling possible by sending messages around the body,110 and Merriam-Webster states that it is the bodily system that in vertebrates is made up of the brain and spinal cord, nerves, ganglia, and parts of the receptor organs and that receives and interprets stimuli and transmits impulses to the effector organs.111 The American Heritage Science Dictionary states that a nervous system is The system of neurons and tissues that regulates the actions and responses of vertebrates and many invertebrates.112
Neuroscience and biology textbooks are more subtle; they define the nervous system as the biological system whose basic cells are neurons.113 However, this definition, too, is phylogenetic since it rests on the premise that only animals have neurons and hence only animals have nervous systems. Also, it is problematic because the literature states two reasons that make it difficult to define neurons by themselves: 1) outside vertebrates and arthropods, there exists no concept of a neuron based on specific features that could define it (e.g., action potentials and specialized synapses are not prerequisites for neurons)114 and 2) genetic analyses have shown that no specific neuronal or synaptic genes exist that are the same in all metazoans.115,116
The nerve-like cellular makeup of plants mentioned in the previous section does not reach the same degree of complexity as animal nerves, but research findings have led some scientists to propose that plants have simple nervous systems and that plant neurobiology exists.4,41 However, these proposals have been openly rejected by some in the scientific community,6,7 who argue that concepts of neuroscience cannot be applied to plants because certain definitions cannot be fulfilled. We think that the emergence of this debate puts the spotlight on the definitions used in neuroscience, specifically on the definition of a nervous system.
As previously mentioned, this controversy could be considered an ontological debate, but we claim that this assessment is erroneous because phylogeny-based definitions of nervous systems impact the field of evolutionary biology. Defining a biological system using a phylogenetic definition determines a set of interrelated elements that carries out a function, and that set is conserved in the species of a specific branch or subtree of the phylogenetic tree. Under a phylogenetic definition, a biological system denotes a phylogenetic tree, and the biological system does not exist outside those species. If we accept a phylogenetic definition, we are denied, de facto, the possibility that the system exists outside the phylogenetic tree stipulated by the definition and that processes of convergence evolution exist. Thus, the phylogenetic definition of a nervous system has shaped the current evolutionary viewpoint to be one in which the nervous system has emerged and evolved only in the animal kingdom.117–119 However, when discussing the evolution of living beings, considering processes of convergence is necessary.8–10 In fact, it is possible that processes of convergence between plants and animals in the signaling system exist,98, and it should at least be discussed.
An alternative to phylogenetic definitions for biological systems is functional definitions. Several biological systems are defined using physiological criteria (e.g. the digestive, transport, respiratory and reproductive systems). When considering the evolution of the respiratory system in living organisms, we can use physiological criteria to compare its evolution in insects, mammals, and fish, even though the anatomical elements and mechanisms of the systems are different.120 We can also compare the respiratory systems of plants and animals,121 but assuming a phylogenetic definition automatically affects evolutionary biology because it excludes comparing the evolutionary paths of the nervous system in plants and animals. This difference in how we discuss the nervous system and other biological systems shows how limiting a phylogenetic definition is.
It is also relevant to discuss the phylogenetic definition of a biological system in the framework that systems theory proposes. One of the hallmarks of systems theory’s framework is that a system is characterized by mathematical functions.122,123 On the basis of that fact, if a system is characterized by the function and another is characterized by the function , they are determined to be the same, if for each state, , the systems satisfy . Also, the functional viewpoint of systems theory implies that the physical nature of the elements that constitute the system is not important in determining what kind of system it is. If it is applied to biological systems, we can address the existence of the same biological system in different species only on the basis of the function it performs. For example, the biological systems that carry out respiratory functions are considered respiratory systems, even though they differ regarding the elements and organs they possess. Because a phylogenetic definition precludes considering the existence of the same biological system in different species without an ancestor–descendant relationship, it clashes with system theory and the physiological point of view.
At this point, someone could counterargue that using a physiological definition to identify a nervous system might not be necessary because there are no nervous systems in other kingdoms or phyla to compare and discuss. However, investigations in plants have found long-distance electrical signals that control and activate different functions, a fact which, from a physiological viewpoint, must be associated with a biological system. The existence of features, or elements, in the electrical signal systems of plants and animals about which evolutionary biology must answer about each one whether it is a homologous or an analogous feature, or element, would imply the necessity of a broader definition of a nervous system to elaborate an answer. Following this premise, we have identified three specific physiological issues that imply the necessity of a broader definition: 1) electrical signals and their mechanisms, 2) cell-to-cell mechanisms to propagate electrical signals and 3) parallelism between the animal autonomic nervous system and plant nervous system. Next, we discuss each issue.
1. Electrical signals and their mechanisms. Electrical signals have been observed in animals and plants. The most striking case of having similar electrical signals is the action potential, also called a spike, which is characterized by giving a maximum response or none at all. In animals, there are two kinds of action potentials: sodium-dependent and calcium-dependent.124 Both types are generated by voltage-gated ion channels that regulate ion flow across the membrane in response to the membrane potential. Sodium-dependent action potentials are produced by currents of and and calcium-dependent action potentials are produced by currents of and . Calcium-dependent action potentials were first discovered in mammals in Purkinje cell dendrites,125,126 and currently we know they are in pyramidal neurons in layer 5 and play an important role in behavior and cognitive function.127 and action potentials are generated through voltage-gated ion channels, but whereas there is only one kind of action potential, there are two kinds of calcium-dependent action potentials: low-threshold and high-threshold.20,128 High-threshold action potentials are initiated by high-threshold channels and low-threshold action potentials by low-threshold channels. Specifically, dendritic spikes in Purkinje cells are dependent on the P-type calcium channel.129 Although the action potential recorded in Purkinje cell dendrites and the low-threshold calcium spike observed in central neurons are both all-or-nothing signals, their electrophysiological properties are different. The action potentials in plants are believed to be produced by fluxes of , and .130 The existence of voltage-dependent channels has been proven,55,131 but the channels involved in the currents for the depolarization in action potential in plants have not yet been identified. Also, we know that some action potentials have a complex mechanisms in some plants. For example, in Chara corallina, the action potential is not based only on voltage-dependent ion channels; the exposure to light causes a progressive shift in the depolarization maximum.132 But this complexity does not signify a difference between plants and animals because some neurons in animals also have action potentials that are not entirely based on time- and voltage-dependent ion channels.133 A recent study in Nitellopsis obtusa and Marchantia polymorpha has shown that inhibitors of human two-pore channels alter resting potential, action potential amplitudes and the duration of action potential repolarization by affecting channels.134
In addition to having a short-lasting depolarisation of the action potential, animals and plants both have signals based on prolonged depolarisation. In animals, a prolonged depolarisation signal is the plateau potential,135,136 and in plants it is the variation potential.2 The plateau potentials began to be studied in the neurons of mantis shrimp.137 Later, they were discovered in mammals in Purkinje cells dendrites,126 and currently, plateau potentials are far from being considered a rarity. Another remarkable coincidence regarding the mechanisms plants and animals use in electrical signals is that plants use ATPases in electrical signalling, like animals do. ATPases are key molecular structures that maintain the ionic gradients in animals’ neural cells.138 Research on the Arabidopsis confirms that ATPases play an important role in regulating membrane repolarisation in wound response.58 It is foreseeable that additional research in the next few years will further clarify the molecular mechanisms of electrical signals in plants, and once that is completely understood, we should discuss whether a process of evolutionary convergence exists.
2. Long-distance signals. Different studies have shown that plants have mechanisms to generate, transmit and process electrical signals, that their electrical activity contains information about environmental stimuli,51,52 and that action potentials transmit information.50 In the animal nervous system, two kinds of processes have emerged to send long-distance signals: electrochemical and electrical coupling. Regarding plants, many molecular details are still unknown about how they perform cell-to-cell communication, but we know they use neurotransmitters, such as glutamate and GABA. We know that plants have not developed synapses, but it is unknown whether any convergence exists with the volume transmission in animals.139,140 Another option is that plants perform cell-to-cell communication through electrical coupling. One hypothesis is that electrical coupling communication could happen through plasmodesmata. Electrotonic coupling was thought to be only a minor feature of the nervous system of less evolved animals until it was also discovered in mammals,141,142 and we now know that it is an important mechanism in different central nervous system structures, such as the inferior olive.143 The first direct proof of electrical coupling in plants was found in the Elodea canadensis,144 and electrical coupling between root cells is involved in how plants can extract water from dry soil against a gradient in water potential.145 Evidence of electrical coupling in electrical circuits has also been found in the Bidens pilosa L., Venus flytrap146 and Aloe vera.147 Investigations of electrical networks in Dionaea and Aldrovanda trap lobes have revealed a high, rich synchronous coupling activity. Action potentials spread rapidly enough to adjacent cells in the trap lobes to allow the plant to catch prey.148 Indirect evidence shows that the cells may be coupled bidirectionally, making them fire synchronously in aquatic carnivorous plants.149 Given this evidence, the evolutionary history of animals and plants cannot be described without discussing whether a process of evolutionary convergence has generated the same mechanism to propagate electrical signals in multicellular organisms.
3. The autonomic nervous system. In the peripheral nervous system of animals, we find the autonomic nervous system (formerly named “vegetative nervous system”), which controls smooth muscle and glands and thus guides the function of internal organs.150 The autonomic nervous system monitors arterial pressure, the concentration levels of different substances in the blood, such as carbon dioxide, oxygen and sugar, and the chemical composition of the stomach and gut content. Depending on the values registered, the autonomic nervous system affects functions such as heart rate, digestion, respiration, pupillary response, urination and sexual arousal, among others. In plants, photosynthesis, respiration, phloem transport2 and ovarian metabolism151 are regulated by electrical signals. Plants have mechanisms that, depending on the levels of substances or physical variables, send electrical signals to regulate the functions mentioned. Also, plants use electrical signals to affect hormone synthesis regulation.152,153 In animals, the interaction between the automatic nervous system and the endocrine system is a current research topic,154 and one cannot avoid wondering to what extent parallels can be drawn with this topic too. Since similar functions are regulated by the autonomic nervous system in animals and by electrical signals in plants, it is necessary to discuss whether there is a process of evolutionary convergence between both biological systems.
At this point, we consider it relevant to review the work of Darwin, the father of the theory of evolution. In his work, we find arguments against a phylogenetic definition of the nervous system. Darwin wrote three books about plant life,155–157 which is not a coincidence – he was trying to change our view on plants. He wrote, “It is a truly wonderful fact – the wonder of which we are apt to overlook from familiarity – that all animals and all plants throughout all time and space should be related to each other in group subordinate to group.” [158, p. 155]. Darwin understood that evolution involves a global view about all living beings that requires a framework in which all evolutionary pressures, directions and paths can be formulated and compared. He was able to see that plants should not be isolated from animals in the study of evolutionary processes. Also, he wrote the following:
“I am inclined to believe that in nearly the same way as two men have sometimes independently hit on the very same invention, so natural selection, working for the good of each being and taking advantage of analogous variations, has sometimes modified in very nearly the same manner two parts in two organic beings, which owe but little of their structure in common to inheritance from the same ancestor.” [158, p. 193].
Clearly, Darwin’s work shows he rejected the phylogenetic definition of a biological system because he was aware of the existence of convergence processes.
We also consider it relevant to review the work of Ramón y Cajal, the father of modern neuroscience, in regard to a phylogenetic definition of the nervous system. Although we are not aware Ramón y Cajal had any interest in studying plants, he considered that each nervous system must be understood in the context of the evolutionary and ethological niche in which it has developed and survived.159 Therefore, it can be claimed that the emergence of a nervous system in plants agrees with Ramón y Cajal’s view since plants’ nervous systems must be understood in their corresponding contexts and the plants’ evolutionary and ethological niche differs from the animals’. Once again, some could claim that neurons and synapses do not exist in plants,6 and certainly Ramón y Cajal’s work is deeply linked with the concept of synapsis, but we have two objections to that claim. First, although the plant cells that transmit electrical signals do not have synapses, using the existence of synapses to define a neuron is not currently a requirement in the field of neuroscience,160 and from the evolutionary viewpoint, “[n]either action potentials nor specialized synapses are absolute prerequisites of neurons” [114, p. 186]. Some neurons have graded potentials instead of action potentials to transmit information.161 In animals, neurons exist with different kinds of chemical synapses,162 and another kind of intracellular transmission different from wired transmission has even been found, called volume transmission (also named non‐synaptic diffusion neurotransmission).139,163 Second, Ramón y Cajal’s neuron doctrine,164,165 which he based on much careful research, asserts that the nervous system, in keeping with Schleiden and Schwann’s broader cell theory, is composed of discrete and specialized cells in sending signals. In his view, the special features of nerve cells (e.g., axon, synapses) correspond to evolutionary adaptations associated with niche specialization. According to Ramón y Cajal, being composed of specialized cells is the fundamental criterion for defining a nervous system, and how, and how the cell is specialized (e.g., axon, synapses) corresponds to the evolutionary process and ecological niche. Although an anatomical feature is sufficient for being a specialized cell, there is no specific feature necessary to specialize in transmitting, processing or generating signals. Therefore, the key issue in the definition of a nervous system from Ramón y Cajal’s neuron doctrine is that a nervous system is made up of specialized cells, and it does not support a phylogenetic definition of the nervous system.
Evolutionary arguments at the molecular level also support adopting a physiological definition to identify nervous systems. Electrical signaling is based on the molecular mechanisms of the ion channels and pumps in both plants and animals, a fact that did not occur by chance. Genetic research has shown that there are no de novo structures in living beings but only those that have evolved from preexisting structures.166 We also know that a basis for ion channels already existed in the prokaryotes,167 and some researchers have focused on the relationship between signaling and the evolution of ion channels in plants and animals.168 Those studies show that issues about the evolutionary path of the molecular signaling mechanisms are often studied and discussed from an evolutionary viewpoint. Additionally, ligand-gated ion channels are ancient,169 and the emergence of GABA and glutamate mechanisms is an ongoing research topic. Analyses indicate that the glutamate-binding mechanisms of plants and animals have an ancestral glutamate-binding mechanism as a common origin, and they have been diverging.169 In animals, glutamate receptors with a high ligand specificity have been selected, whereas in plants glutamate receptors have evolved to be nonspecific amino acid sensors.170,171 In parallel to that divergent process, the specificity of the glutamate mechanism emerged in animals through a convergent process.172 In contrast to the divergent process that occurred in plants and animals regarding glutamate receptors, the existence of an anion channel inhibited by GABA is due to a convergent process in plants and animals.173
Regarding these evolutionary analyses, if one can discuss the evolution of molecular signaling mechanisms,169,174 it is illogical that one is unable to discuss the evolution of the biological systems that contain those molecular mechanisms. Although at the biological system level they do not share a phylogenetic tree, they do share one at the molecular–mechanism level. Thus, removing this contradiction is another reason to adopt a new definition to identify nervous systems.
We will now present our stance. Firstly, Bose’s initial claim stating that “the physiological mechanism of the plant is identical with that of the animal” is wrong because the research clearly shows that the mechanisms are different. On the other hand, Bishop explained, as did Ramón y Cajal, that a variety of nervous systems exist rather than only one kind and that each one uses different properties.175 This is still the current view today.117,176 Thus, although the mechanisms that generate, transmit and process signals in plants are different from animal nervous systems, there is no a priori reason to deny that they constitute nervous systems that are within the variety of nervous systems that exist in nature. We position ourselves with those who claim that speaking about plant neurobiology is reasonable. We do not claim, however, that the terminology for plant neurobiology must be equal to or based on the terminology that already exists for animal neurobiology. Given these clarifications, what options are available to address this dispute? One attempt to resolve the dispute was made by Peter W. Barlow. He proposed using the Living Systems Theory to reinterpret animal and plant neurobiology mechanisms,177 but his proposal did not change either side of the argument. We think that the proposal fails to resolve the dispute for three reasons: it does not identify a specific problem in any of dispute’s positions, it does not propose a solution for any problems in any of the dispute’s positions, and it does not show advantages against the previous position, which would force a change of mind.
Considering these points mentioned here, we have identified problems on each side. On the plant neurobiology side, we consider it impossible to defend or show that plants have a nervous system like that of animals because although plants clearly have a electrical signal system to control and generate their behavior, important differences exist between these systems in plants and animals. On the animal phylogenetic side, we find that the phylogenetic definition of a nervous system, which directly excludes the existence of a nervous system in plants, creates a problem in studying the evolutionary history of animals and plants. This happens because the definition does not allow for addressing whether there have been convergence processes, even though research shows that plants have developed mechanisms to react immediately to exogenous and endogenous states. Also, we find an incompatibility between the phylogenetic definition and the general system theory. Since discussing convergent evolution processes of a system or an organ in species that are phylogenetically far removed requires identifying the system or organ that carries out a specific function in those organisms, our proposed solution is to broaden the definition of a nervous system by adopting a physiological definition. We propose this solution because a physiological criterion characterizes a system by its function and is therefore also compatible with systems theory, and the variety of electrical signals and molecular mechanisms that generate them in animals leads us to understand that the definition of a nervous system cannot be formed by identifying a specific mechanism.
In the following sections, we develop our proposal to broaden the definition of a nervous system and show that this solution provides a framework to generate hypotheses about the evolutionary history of animals and plants that cannot be formulated using the phylogenetic definition of a nervous system.
4. Two proposals for broadening the definition of a nervous system
In the previous section, we proposed to broaden the definition of a nervous system by using a physiological criterion to resolve the debate about the existence of nervous systems in plants. But how can we do this? The definition has already been broadened by modifying the neuron doctrine by explicitly adding features to the definition of a neuron.160 However, that broadening has led to the Eumetazoa’s nervous system cells remaining under the phylogenetic definition after the unexpected discoveries made in the 20th century (e.g. gap junctions, neuromodulatory substances). Being against using phylogenetic definitions, we defend that the fulfillment of the definition of a biological system cannot be contingent upon belonging to a kingdom or phylum; one should not reject that a biological system belongs to a class of biological systems based on the mere fact of it not belonging to a kingdom or phylum and without studying the system’s function. Regarding this position, we suggest two options for broadening the definition of a nervous system. The first option applies to the domain of multicellular organisms. The second one applies to systems, so it is more general than the first and can be applied to unicellular organisms. We develop these two options below.
4.1. Broadening the definition for multicellular organisms
Because using specific anatomical features to define a neuron has been shown to be a complex issue in evolutionary biology,114 we propose avoiding any phylogenetic or anatomical references and considering mainly physiological criteria to formulate the definition. The basic functional unit of all known living organisms is the cell, and Theodor Schwann proposed that tissues are groups of cells that work together to carry out a specific function.178 Ramón y Cajal found that nervous tissue, the main tissue of the nervous system, is made up of cells.164 Our modern view on the nervous system’s function is that it transmits, generates and processes information to increase the probability of future beneficial situations (including avoiding damage) for the organism.16 Combining all these points, we propose that a nervous system can be defined as follows:
A nervous system is the system of a multicellular organism that (1) contains a group or groups of cells that are specialized in transmitting, generating or processing information, (2) sends signals to other systems, allowing the organism to react to or act upon exogenous and endogenous states by controlling those systems’ activity, and (3) generates and sends signals to other systems as the result of communication among multiple specialized cells of the system.
This definition is likely to raise many questions for the reader, and we address some of these potential questions below.
The use of “communication” and”information” in the formulated definition are not fuzzy concepts that allows subjectivity. We use them in reference to the definitions provided by information theory179 – that is, communication requires that a communication channel exist between one cell and another, and the signal’s value transmit information from the emitter to the receiver cell because it is unknown what the signal’s value will be. We consider that a channel of communication exists only when the transferred element is not an element involved directly in the chemical reactions of the metabolic routes of the receiver cell intended for nutrition. For example, red blood cells that release oxygen do not transmit information because the oxygen is employed directly to create adenosine triphosphate (ATP) in the receiver cell. The glucose molecules transferred from astrocytes to neurons also do not transmit information because they are employed directly to create ATP. However, the neurotransmitters released by neurons in the synapses are not nutrients, and they transport information because it is unknown when the neurotransmitter will be received. Our definition differentiates between nutrients and chemical messengers.
Conditions 2 and 3 are important because, in multicellular organisms, other systems exist that contain cells specialized in transmitting, generating or processing information, but these systems must be excluded from the definition because they do not belong to the class of nervous systems. Condition 2, which determines that a nervous system sends signals only to other systems, excludes systems that have a function that directly affects exogenous or endogenous states, even though these systems have cells that are specialized in transmitting, generating or processing information to carry out their functions. The reason is that, if a system has a function and it evolves by increasing the number of computations to give a better response, such a system is not a nervous system. Also, if a system that transmits, generates or processes information evolves to give a specific response to exogenous or endogenous states by itself, such a system would no longer be a nervous system because the function it performs would have changed. One example of a system that transmits, generates or processes information but does carry out a specific response is the immune system.180,181 The animal immune system is also excluded from the phylogenetic definition of a nervous system.
Regarding Condition 3, we consider it necessary because it establishes a boundary that separates systems that, despite having cells specialized in transmitting, generating or processing information, use approaches that are completely different regarding the signal generation a nervous system carries out. Thus, this condition makes the definition consistent with the computational model of a neural network, and it excludes those systems whose cells carry out their function independently of any other cell of the system, even though they generate, transmit or process information. This does not mean, however, that the computational model of a nervous system can only be the neural network model, because the neural network model determines a specific way of communicating among the cells. Let us look at some specific examples. The first is the animal endocrine system. Its cells carry out processes of generating, transmitting and processing information because cells in glands release hormones to send signals to the organism’s cells that have receptors for them. However, cells in glands do not communicate with other cells to determine which signal is sent: they respond by themselves. The animal endocrine system is also excluded from the phylogenetic definition of a nervous system. This condition is also important because the reader could consider us to have underestimated the processes that plants perform to generate their behavior, but that is not the case. We are aware that plants carry out multiple behaviors to survive, such as managing food reserves,182,183 perceiving the force of gravity to determine growth direction184 and competing successfully for resources, among other behaviors. However, the mechanisms that they use to generate those behaviors are excluded from the definition of a nervous system that we propose because those behaviors are the result of the sum of the behaviors that each cell generates by local computations. Thus, we consider those behaviors either not to belong to a nervous system and to be ascribed to hormonal regulatory systems in plants185,186 or to be generated by an interplay of different systems, as occurs in animals in the neuroendocrine systems, a combination of the nervous and hormonal systems.
This definition of a nervous system implies the existence of a supercategory that contains the category of nervous system. We call this supercategory command-control system.187 It contains the other categories that would cover the systems that generate, transmit or process information but are not nervous systems, such as hormonal systems. However, a discussion of the command-control system category and the other categories it contains is beyond the scope of this paper.
It is key to note that organisms must provide a response to exogenous or endogenous states within a critical time to avoid damage, and this is important because if damage affects the organism’s number of offspring, the endogenous or exogenous states become evolutionary pressures that drive the physical features of the nervous system’s mechanisms. This pressure causes the selection of the organisms that have the speed required to avoid damage. For example, the characteristic electrical signals of a nervous system have emerged directly from the necessity to act and react in real-time and avoid damage. Vertebrates have a vestibular system that contains hair cells that transduce mechanical movements into electrical signals. The speed of registering and transmitting information is fundamental to generating an adequate locomotor response that prevents animals from falling because of gravity. As for plants, they do not move, so they do not need to contend with that problem. In fact, the critical period of time varies from one evolutionary pressure to another, and it allows us to observe a variety of nerve conduction velocities.188,189 Also, the need to synchronize communication among multiple specialized cells produces evolutionary pressures that drive the selection of mechanisms that regulate signal speed.190,191
4.2. Broadening the definition for systems
The previous definition broadened the current definition of a nervous system to allow for establishing whether it is fulfilled for any system of each multicellular organism. However, one could require an even broader definition. For example, creating artificial systems inspired by animal nervous systems192 could require discussing a nervous system in an artificial system. Bishop already stated that “it is not easy to state an intrinsic difference between a nervous system and a computing machine” [175, p. 397]. To achieve this level of generality, it is necessary to have a definition that uses the framework of systems theory. Thus, the definition would be the following:
A nervous system is the subsystem of an autonomous system that (1) contains a group or groups of elements that are specialized in transmitting, generating or processing information, (2) sends signals to other subsystems, allowing the system to react to and act upon exogenous and endogenous states by controlling those subsystems’ activity, and (3) generates and sends signals to other subsystems as the result of communication among multiple specialized elements of the subsystem.
4.3. About discussing the differences between nervous systems
A definition of “nervous system” allows only for determining whether a system belongs to the category defined. “Ordering systems, including classifications, are needed to reduce this chaotic diversity into understandable manageable arrangements before scientific explanations are possible” [193, p. 170]. If we want to discuss evolutionary convergence and divergence, we need to define subclasses within the class of nervous systems, which can be divided in different ways. We propose splitting each definition by using a method to create subclasses that one of the authors of this paper has recently employed.194 This method uses a hierarchy in which each new level breaks the class of nervous systems into more subclasses than the previous one: the higher the level, the greater the number of subclasses into which the nervous systems are split. Each class within each level is split into disjointed subclasses in the subsequent level. One can determine at which level the nervous systems are equal (by establishing that they belong to the same class) and at which level they differ (by establishing that they belong to different classes in that level).
We can obtain different hierarchies to discuss the differences between nervous systems, depending on the nervous system definition selected and the criteria selected to establish the different classes in each level. Even so, there are some features that all hierarchies must share to be useful in their purpose. In all the hierarchies, level must have only one class, the class of nervous systems. To select the criterion of each level, we propose using the following rule. The first level that splits the class of nervous systems must use a physiological criterion, and as the level increases, the criterion changes toward an anatomical criterion.
The following subsection presents a possible hierarchy of the definition for multicellular organisms. It must be noted that a system can also be made of subsystems. Therefore, a nervous system can be made of different subsystems. A hierarchy of the definition for systems can be made by replacing multicellular organism with system and cell with element, but because this hierarchy is similar to the following hierarchy, it is not necessary to include it here.
4.3.1. The hierarchy of levels for multicellular organisms
In level there is only one class, and it contains all the systems considered to be nervous systems by the definition used for multicellular organisms.
Level of the hierarchy contains four subclasses defined from the information theory point of view. The subcategories are the following:
• Generating System: It is one group or several groups of cells that communicate among themselves and generate an output signal that transmits information to other systems.
• Transmission System: It is one group or several groups of cells that communicate among themselves and transport information to other systems.
• Processing System: It is one group or several groups of cells that communicate among themselves and perform a computational process.
• Mixture System: It is several groups of cells that do not all carry out the same type of function from the information theory point of view.
Level of the hierarchy contains eight subclasses defined from the computational point of view. Each previous class is divided into two classes: memory and transitional. We define a memory cell as a unit whose computational power overcomes the computational power of a finite-state machine. A transitional cell is defined as a unit whose computational power is not greater than a finite-state machine; this means that it lacks a memory that allows it to carry out a more powerful calculus than a finite-state machine can perform.195 In this paper, we use the term computational power specifically to refer to the set of functions that a computational model can calculate, and we use capacity of computation to refer to both computational power and the number of computational operations per unit of time that the system can execute. The subcategories in this level are the following:
• Transitional Generating System: It is one group or several groups of transitional cells that communicate among themselves and generate an output signal that transmits information to other systems.
• Transitional Transmission System: It is one group or several groups of transitional cells that communicate among themselves and transport information to other systems.
• Transitional Processing System: It is one group or several groups of transitional cells that communicate among themselves and perform a computational process.
• Transitional Mixture System: It is several groups of transitional cells that do not all carry out the same type of function from the information theory point of view.
• Memory Generating System: It is one group or several groups of memory cells that communicate among themselves and generate an output signal that transmits information to other systems.
• Memory Transmission System: It is one group or several groups of memory cells that communicate among themselves and transport information to other systems.
• Memory Processing System: It is one group or several groups of memory cells that communicate among themselves and perform a computational process.
• Memory Mixture System: It is several groups of memory cells that do not all carry out the same type of function from the information theory point of view.
Level of the hierarchy divides each class from the previous level into two subclasses, interconnected and disconnected, that reference the existence, or not, of independent networks in an organism. For example, Venus flytrap, which has a mixture system in each of its leaf traps is an example of a disconnected transitional mixture system (see Subsection 5.3). Mammals are an example of an interconnected transitional mixture system because their autonomic nervous system comprises three systems: the sympathetic, parasympathetic and enteric.150
Level divides the classes by considering centralized and decentralized transitional systems. A centralized system has an organ-type structure in which its cells carry out the function. A decentralized transitional system lacks such an organ-type structure. In animals, cnidarians are an example of organisms with a decentralized transitional mixture system, and mammals are an example of organisms with a centralized transitional mixture system.
The hierarchy of levels is presented below in Figure 1.
Figure 1.
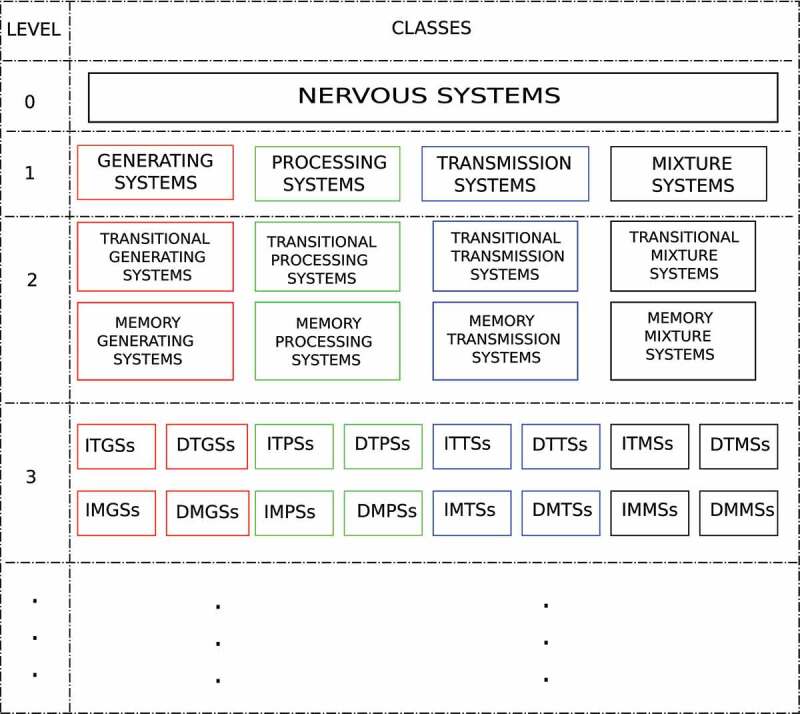
This table shows the different levels and classes into which the nervous system is split. Each class of the level is split into disjointed classes in level . ITGSs = Interconnected Transitional Generating Systems; ITPSs = Interconnected Transitional Processing Systems; ITTSs = Interconnected Transitional Transmission Systems; ITMSs = Interconnected Transitional Mixture Systems; DTGSs = Disconnected Transitional Generating Systems; DTPSs = Disconnected Transitional Processing Systems; DNTSs = Disconnected Neuronal Transmission Systems; DTMSs = Disconnected Transitional Mixture Systems; IMGSs = Interconnected Memory Generating Systems; IMPSs = Interconnected Memory Processing Systems; IMTSs = Interconnected Memory Transmission Systems; IMMSs = Interconnected Memory Mixture Systems; DMGSs = Disconnected Memory Generating Systems; DMPSs = Disconnected Memory Processing Systems; DMTSs = Disconnected Memory Transmission Systems; DMMSs = Disconnected Memory Mixture Systems
The discussion about convergence and divergence does not have to stop at the fourth level; the classes of the fourth level can be split using physical features, but the shape generated by the splitting would be a tree of levels instead of a hierarchy of levels.
5. Discussing the directions of nervous system evolution in plants and animals
We have claimed that broadening the definition of a nervous system is beneficial to studying and discussing the evolution of generating, transmitting and processing signals in living beings. Now, we would like to provide specific examples of evolutional issues that emerge when this broadening is assumed.
5.1. The evolution of capacity of computation
One of the most striking differences between the nervous systems of plants and animals is the capacity and the kind of computation that each one possesses. At this point, the reader must remember that our definition contemplates only one specific type of system within all the systems that organisms possess to process, generate and transmit information. The plant nervous system is fundamentally dedicated to transmitting information, and it carries out a low level of information processing, even in carnivorous plants (e.g., the Venus flytrap) – this is true even if plants can process information in the root apex transition zone. Unlike in plants, the animal nervous system carries out mainly information processing; even reflex arcs are circuits that process information to generate behavior. Therefore, the evolutionary pressures have made plants’ and animals’ nervous systems evolve following two divergent paths. The animal nervous system has evolved to process information and the plants nervous system has evolved to transmit information.
According to the free-moving hypothesis,15,194 animals need to move constantly to acquire nutrients because the nutrients available at each point in the environment are limited. Moving requires decision-making about which directions are best to pursue because a natural environment is a heterogeneous space wherein the features of one point in space can differ greatly from another: the choice of direction can lead to very different consequences for the organism. Therefore, animals face a selective pressure to be motile to acquire resources, and this requirement generates a selective pressure to carry out real-time information processing to find nutrients and avoid danger. In contrast, most plants are sessile organisms that convert inorganic matter into chemical energy using photosynthesis. Because environments are common in which sunlight is available throughout the entire day, plants do not face the same selective pressure that animals do to move to acquire nutrients. The pressure placed on plants has occurred in response to abiotic and biotic stressors in their locations.196 Considering this fact, plants mainly need a nervous system to detect stressors and transmit signals to cells so that they can generate a response within the critical period of time.
5.2. The emergence of neural polarity and chemical synapse
A neuron is a highly polarized cell that generally has a long axon and several short dendrites. Alongside morphological neural polarization, the mechanism of chemical synapse has an outstanding role in all animal nervous systems. The mechanism of neural polarity has been intensely studied,197 as well as the chemical synapse,198,199 but the evolutionary reasons for their selection are a matter of debate.200,201 Here, we address these reasons using the framework that arises from our definition.
Three hypotheses have been proposed to explain why the axon emerged: to transmit information over long distances between sensor cells and effector cells,202–204 to perform internal coordination,205 and to reduce the sensory cells used in signal transmission.206
Because plant nervous systems have evolved to transmit signals and can transmit information over long distances between sensor and effector cells,2,64,80 one would expect plants to have developed axons if one or more of the previous hypotheses were true. However, plant axons do not exist; plants have alternative mechanisms to transmit electrical signals to distant points.67 The multiple studies that show that plants send electrical signals from one point to another prove that the axon did not emerge because of the need to send long-distance signals: this type of transmission is possible without an axon, so the explanation for why axons and synapses emerged in the animal nervous system lies elsewhere. To work toward finding this explanation, we have compared signal transmission in plant and animal nervous systems, and propose the following reasoning:
Because plant nervous system cells can communicate signals without having developed axons42,45,65 and animal nervous system cells have developed and maintained axons to transmit information,207 we can ask the following question: What is the main difference in function between the axons of animal nervous system cells and the mechanism of repetition points in plants? Our answer is the speed of signal transmission. In axons, signal speed is in the order of magnitude of meters per second,113 while in the phloem of a plant it is millimeters65 or centimeters per second.38,39 As we mentioned in Section 4.1, the duration of the critical period to respond is an evolutionary pressure. Thus, our hypothesis is that the axon was selected in animals because it achieves the speed required to adapt to the environment, and this speed cannot be achieved with the mechanism selected in plants. This limitation is not a problem for plants because the critical period of time that affects the evolution of their signaling mechanism is longer than it is in animals, so the mechanism selected in plants requires only the speed needed to fulfil the critical period of time of plants. Also, other evolutionary pressures which differ in animals and plants (for example, energetic restrictions) would have caused the selection of the system of repetition points without axons that plants possess.
This would explain why axons have been selected in animals, but not why dendrites and synapses have also been selected. Axons and volume transmissions in animals can send signals over long distances,163,208 so it does not seem reasonable that nature has selected dendrites and chemical synapses to perform the same task in the same nervous systems. Again, we must ask another question: In what way does the polarity of nervous system cells (axons, dendrites) and synapses in animals contribute to the function that a nervous system carries out? One initial hypothesis could be that animals need information about the location of a signal’s origin because their response is often to activate muscle tissue where the signal originated (e.g., reflex arc), and plants do not need to transmit the location of the signal’s origin because the signal is used to generate a systemic physiological response. However, plants such as the Mimosa pudica and the Venus flytrap use signals to generate responses in specific locations; their anatomical organization allows for those responses, despite the plants not knowing the origins of the signals. Thus, retaining signal origin information to generate responses in specific locations is not sufficient to explain why neural polarity and chemical synapses have been selected in animals.
Discarding the hypothesis of generating responses in specific locations, we propose a new explanation for why conserving signal origin information has been a pressure that has driven neural polarity and synapses to emerge as features of the nervous system in animals. Synapses are commonly understood to be mechanisms highly specialized in cell-to-cell communication, but in addition to that, they allow information about signal origin to be retained implicitly. We propose that neural polarity and chemical synapse emerged because conserving information about signal origin is a necessity in the computational model of the neural network. On top of chemical synapse sending signals, its role in the computational process of a neural network would be conserving signal origin information. Specifically, the computational process of a neural network requires weighing each signal depending on its origin. The concept of weighing in the computational model of a neural network is equivalent to the concept of synaptic strength found in the animal nervous system. We already mentioned in 5.1 that animals’ need for computation has been an evolutionary pressure, and animals’ need for computational robustness would have been an evolutionary pressure that caused the computational model of a neural network to be selected.194
If we analyze the communication mechanisms of the plant nervous system cells from a functional point of view, we observe that they either alter the local chemical environment registered by nearby cells or are coupled through plasmodesmata to transmit electrical signals.64 These mechanisms do not allow plants to carry out complex computational processes because neither conserves information about signal origin; they only allow plants to transmit electrical signals to react to the environment, but they are sufficient for adapting to the environment.
Based on the above, we propose that neural polarity and chemical synapse are elements of the same mechanism and that this mechanism was selected not because it transmits signals over long distances but because it allows for implementing the neural network model and carrying out computational processes. The idea of neural polarity and chemical synapse as one mechanism explains that evolution has produced dendro-dendritic, axo-axonal and reciprocal synapses because they are variations of this mechanism that boosts computing capacity. Thus, although neural polarity, volume transmission and chemical synapse seem to be three mechanisms in the animal nervous system, these three elements have given rise to two different mechanisms: axon-volume transmission and neuronal polarity-chemical synapse. The existence of two mechanisms whose functions are clearly different obliges us to consider the evolutionary pressures that have caused each to evolve. In turn, the evolutionary pressures of each element of each mechanism must be analyzed regarding whether the element is integrated into one mechanism or the other because the function of each mechanism is different. For example, the evolutionary pressures that caused the axon to be selected could be different in each of the mechanisms. This implies that the existence of a similar element in both mechanisms may not be due to a conservation processes and the same evolutionary reasons. Thus, analyzing the evolutionary reasons the neuron exists must be done while regarding which mechanism it is integrated into. This conceptual framing is compatible with the hypothesis of the exaptive origin of chemical synapses,209 and it should be taken into account in the hypothesis of the independent origins of neurons and synapses and the analysis of each origin.115,116
5.3. A centralized nervous system, decentralized nervous system and disconnected nervous system
Another issue is centralized, decentralized, and disconnected nervous systems in multicellular organisms. Each option is a different evolutionary path for nervous systems. We can find centralized nervous systems in multicellular organisms that need global coordination and a high computational capacity. If global coordination requires a high capacity for computation, then a pressure exists to evolve toward a centralized nervous system so that energetic and temporal costs can be reduced.210 If all the computation is centralized, there is also a pressure to protect the computational structure because it would not be energetically possible to have several structures with a high computational capacity to substitute for another when it fails. However, if the nervous system is decentralized, localized damage has a lower impact on the system because it can affect only a few of the system’s elements, and the organism has a high probability of surviving without the cost of requiring additional structures to protect the nervous system (e.g., cranial bones).
On the other hand, if a high computational capacity is not required, a more distributed system can exist. For example, the echinoderm nervous system is decentralized, and there is evidence that different parts of the nervous system can coordinate whole animal behavior.211,212 In plants, the nervous system consists of different electrical circuits.213 The Venus flytrap’s nervous system is an example of a disconnected nervous system because it has a transitional processing system in each of its leaf traps. The advantage of local processing is that the organism does not have to spend energy creating a structure to protect itself because damage to one leaf trap does not compromise the organism’s existence, as the loss does not affect the remaining leaf traps. In disconnected structures, this organization, which performs local information processing, minimizes the effects of damage with the lowest energetic cost.
By combining our analyses of animal and plant nervous system organizational processes, we can conclude that the requirement for global coordination affects the evolution of nervous systems, causing the selection of centralized nervous systems. If no global coordination at all is required, a system can be organized to have isolated computational subsystems, and this option is selected because it minimizes the effects of damage to the system with the lowest energetic cost.
If no global coordination at all is required, a system can be organized to have isolated computational subsystems, and this option is selected because it minimizes the effects of damage of the system with the lowest energetic cost.
6. Conclusions
Several botanists have defended the existence of a nervous system in plants. However, this idea is not well accepted by other scientists.6,7 If the nervous system is defined as the part of an animal’s body that coordinates its actions and transmits signals to and from different parts of its body, then obviously the discussion about the evolution of the nervous system in plants cannot exist. One could consider this debate to be just a terminological dispute, but the definition of a nervous system is not an inconsequential issue about terminology. In this paper, we have shown that the current definition of a nervous system has negative consequences in the field of evolutionary biology that preclude discussing the processes of convergent evolution in multicellular organisms. A phylogenetic definition of an organism’s biological system prevents us from considering whether that system has emerged in other organisms outside that definition. Determining that one biological system of an organism and another biological system of a different organism do not belong to the same kind because they do not share a phylogenetic tree is an error because evolution has taught us that a similar evolutionary pressure to fit can cause the emergence of similar traits in phylogenetically distant organisms. Also, it does not seem logical that one can discuss the evolution of molecular mechanisms because we can trace the phylogenetic trees for them, but not discuss the evolution of the biological systems that contain those molecular mechanisms. In this article, we have defended the necessity of removing the criterion that circumscribes the definition of a nervous system to animals because it precludes discussing the historical evolution of plants and animals by not allowing for addressing whether a trait is similar due to homology or homoplasy.
The academic conflict between those who defend and those who deny the existence of a nervous system in plants comes from the question of whether it is valid to use the definitions made by neuroscience through the study of the Eumetazoa clade in species of another kingdom. We do not think that discussing whether the Eumetazoa’s definition of “nervous system” can be applied to the kingdom Plantae can solve the dispute. We have proposed here an alternative solution: broadening the definition of “nervous system” by using physiological criteria. We can find the use of physiological criteria in the definitions for other biological systems, and using this kind of criteria for nervous systems would allow for discussing evolutionary convergence processes between plants and animals, which is a necessity in the field of evolutionary biology. On the basis of these arguments, we have developed two broader definitions for a nervous system: one focuses on multicellular organisms and the other on the broader requirement of defining a system only by its function. Also, we have shown, in Section 5, that assuming a broader definition allows for a deeper discussion of the evolution of transmitting and processing environmental signals in plants and animals, since it opens up the possibility of formulating new evolutionary hypotheses and reasoning about evolutionary scenarios. Plant nervous systems can be a key element of discussing signal transmission in the animal nervous system. Thus, our proposal not only serves as a possible solution to this dispute but also creates a framework in which new issues about the evolution of signaling systems in different kingdoms can be raised.
Research has clearly shown that there are species in the kingdom Plantae with systems that use electrical signals to transmit information from one place to another of the organism50,60,214 and their electrical activity contain information about the environmental stimuli.51,52 From an evolutionary viewpoint, biological systems emerge if they provide an evolutional advantage that increases the fitness value of species to their niches. The fact that plants locally perceive signal transmissions from distant points within their bodies allows them to respond to future adverse situations in parts of the organism that have not yet suffered damage. Experiments have shown that this ability increases the fitness of the plants that have it over those that do not. Therefore, a signal system emerging in plants is consistent with the theory of evolution. By examining the functional equivalence among niches of plants and animals, we could study and discuss the effect of each evolutionary pressure on the appearance of convergence in the evolution of signal transmission and processing. It is important to know the effects of each evolutionary pressure and how they have driven the evolution of the nervous system.
After presenting the important arguments within the field of evolutionary biology that support our proposal to broaden the definition of a nervous system presented, we claim that textbooks’ current phylogenetic definitions of a nervous system impede obtaining a complete view of plant and animal evolution. Textbooks should use a physiological definition that considers the system’s function. We are aware that modifying a definition is a thorny subject, but we believe our reasoning is sound and the facts sufficient for taking this step. Science must use a framework that does not exclude any empirical evidence in the formulation and verification of hypotheses. Also, this would not be the first time science redefines a concept or even a field (consider the history of organic chemistry,215 for example). Recovering the Dobzhansky’s claim “Nothing in biology makes sense except in the light of evolution”216 and it includes a nervous system. Therefore, to advance our knowledge of the nervous system, we should adopt a physiological definition.
Acknowledgments
We would like to thank Prof. Francisco J. Rubia Vila for encouraging us to continue working after our first draft, Dr. Javier Yajeya Pérez for discussing electrophysiology issues with us, Dr. Timothy Peter Lynagh for speaking with us about the evolution of glutamate receptors in plants and animals, and the anonymous reviewers for their careful analysis, which helped us improve this article. We are also grateful to Lori-Ann Tuscan for assisting with language editing.
References
- 1.Erulkar S, Lentz T (2019). Nervous system. Accessed: 2019 July 15. https://www.britannica.com/science/nervous-system
- 2.Sukhov V, et al. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog Biophys Mol Biol. 2019;146:1–18. [DOI] [PubMed] [Google Scholar]
- 3.Baluška F, Mancuso S. Plants and Animals: Convergent Evolution in Action?. Plant-Environment Interactions: From Sensory Plant Biology to Active Plant Behavior. 2009;4(12):285–301. Berlin: Springer. doi: 10.4161/psb.4.12.10574. [DOI] [Google Scholar]
- 4.Brenner E, Stahlberg R, Mancuso S, Vivanco J, Baluška F, Van Volkenburgh E. Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci. 2006;11(8):413–419. doi: 10.1016/j.tplants.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Iriti M. Plant neurobiology, a fascinating perspective in the field of research on plant secondary metabolites. Int J Mol Sci. 2013;14:10819–10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpi A, Amrhein N, Bertl A, Blatt MR, Blumwald E, Cervone F, Dainty J, De Michelis MI, Epstein E, Galston AW, et al. Plant neurobiology: no brain, no gain? Trends Plant Sci. 2007;12(4):135–136. doi: 10.1016/j.tplants.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Struik P, Yin X, Meinke H. Plant neurobiology and green plant intelligence: science, metaphors and nonsense. J Sci Food Agric. 2008;88:363–370. [Google Scholar]
- 8.Blount ZD, Lenski RE, Losos JB. Contingency and determinism in evolution: replaying life’s tape. Science. 2018;362(6415):6415. doi: 10.1126/science.aam5979. [DOI] [PubMed] [Google Scholar]
- 9.Conway Morris S. Evolution: like any other science it is predictable. Philosophical Transac Royal Soc London B: Biol Sci. 2010;365(1537):133–145. doi: 10.1098/rstb.2009.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vermeij G. Historical contingency and the purported uniqueness of evolutionary innovations. Proc Nat Acad Sci. 2006;103:1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo P, et al. Integrated information as a possible basis for plant consciousness. In: Biochemical and Biophysical Research Communications, (in press). in press; 2020. [DOI] [PubMed] [Google Scholar]
- 12.Taiz L, et al. Plants neither possess nor require consciousness. Trends Plant Sci. 2019;24(8):677–687. [DOI] [PubMed] [Google Scholar]
- 13.Taiz L, et al. Reply to trewavas et al. and calvo and trewavas. Trends Plant Sci. 2020;25(3):218–220. [DOI] [PubMed] [Google Scholar]
- 14.Trewavas A, et al. Consciousness facilitates plant behavior. Trends Plant Sci. 2020;25(3):216–217. [DOI] [PubMed] [Google Scholar]
- 15.Llinás R (1987). Mindwaves, chapter “Mindness” as a Functional State of the Brain, 339–358. Oxford. [Google Scholar]
- 16.Llinás R. I of the vortex: from neurons to self. MIT press, Cambdrige, Massachusetts; 2001. [Google Scholar]
- 17.Galambos R, Makeig S, Talmachoff P. A 40-hz auditory potential recorded from the human scalp. Proc Nat Acad Sci. 1981;78:2643–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llinás R, et al. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28(6):325–333. [DOI] [PubMed] [Google Scholar]
- 19.Llinás RR, et al. γ-Band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Nat Acad Sci. 2007;104(45):17819–17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llinás R, Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981;315:569–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Binder M. Encyclopedia of Neuroscience. Berlin: Springer; 2009. others (Eds.). [Google Scholar]
- 22.Williams SE, Pickard BG. The role of action potentials in the control of capture movements of Drosera and Dionaea. In Plant Growth Substances. 1980; Vol. 1979. 470–480. [Google Scholar]
- 23.Burdon-Sanderson J. Note on the electrical phenomena which acompany irritation of the leaf dioanea muscipula. Proc R Soc London. 1876;25:411–443. [Google Scholar]
- 24.Bose J. Comparative electro-physiology, a physico-physiological study. Longmans Green, Calculta; 1907. [Google Scholar]
- 25.Bose J. Researches on Irritability of Plants. Longmans Green, Calculta; 1913. [Google Scholar]
- 26.Bose J. The nervous mechanism of plants. Longmans Green, Calcuta; 1926. [Google Scholar]
- 27.Hedrich R, Neher E. Venus flytrap: how an excitable, carnivorous plant works. Trends Plant Sci. 2018;23:220–234. [DOI] [PubMed] [Google Scholar]
- 28.Volkov A. Signaling in electrical networks of the Venus flytrap (Dionaea muscipula Ellis). Bioelectrochemistry. 2019;125:25–32. [DOI] [PubMed] [Google Scholar]
- 29.Yang R, et al. A mathematical model on the closing and opening mechanism for venus flytrap. Plant Signal Behav. 2010;5(8):968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherzer S, et al. Venus flytrap trigger hairs are micronewton mechano-sensors that can detect small insect prey. Nat Plants. 2019;(5):670–675. [DOI] [PubMed] [Google Scholar]
- 31.Suda H, et al. Calcium dynamics during trap closure visualized in transgenic venus flytrap. Nat Plants. 2020;(6):1219–1224. [DOI] [PubMed] [Google Scholar]
- 32.Burri J, Saikia E, Läubli NF, Vogler H, Wittel FK, Rüggeberg M, Herrmann HJ, Burgert I, Nelson BJ, Grossniklaus U, et al. A single touch can provide sufficient mechanical stimulation to trigger venus flytrap closure. PLoS Biol. 2020;18(7):1–19. doi: 10.1371/journal.pbio.3000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masi E, et al. Spatiotemporal dynamics of the electrical network activity in the root apex. Proc Nat Acad Sci. 2009;106(10):4048–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed-Guy S, et al. Sensitive plant (Mimosa pudica) hiding time depends on individual and state. PeerJ. 2017;5:e3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibaoka T. Excitable cells in Mimosa. Science. 1962;137:226. [DOI] [PubMed] [Google Scholar]
- 36.Volkov A, Markin V. Active and passive electrical signaling in plants. Prog Botany. 2015;76:143–176. [Google Scholar]
- 37.Hagihara T, Toyota M. Mechanical signaling in the sensitive plant. Mimosa Pudica L Plants. 2020;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkov A, et al. Signal transduction in mimosa pudica: biologically closed electrical circuits. Plant Cell Environ. 2010;33(5):816–827. [DOI] [PubMed] [Google Scholar]
- 39.Volkov A, et al. Circadian rhythms in electrical circuits of clivia miniata. J Plant Physiol. 2011a;168(15):1753–1760. [DOI] [PubMed] [Google Scholar]
- 40.Volkov A, et al. Morphing structures and signal transduction in mimosa pudica l. induced by localized thermal stress. J Plant Physiol. 2013b;170(15):1317–1327. [DOI] [PubMed] [Google Scholar]
- 41.Oyarce P, Gurovich L. Evidence for the transmission of information through electric potentials in injured avocado trees. J Plant Physiol. 2011;168:103–108. [DOI] [PubMed] [Google Scholar]
- 42.Shimmen T. Electrical perception of “death message” in chara: involvement of turgor pressure. Plant Cell Physiol. 2001;42:366–373. [DOI] [PubMed] [Google Scholar]
- 43.Stahlberg R et al.(2006). Communication in Plants: neuronal Aspects of Plant Life, chapter Slow Wave Potentials — a Propagating Electrical Signal Unique to Higher Plants, 291–308. Springer-Verlag Berlin Heidelberg. [Google Scholar]
- 44.Szechyńska-Hebda M, et al. Electrical signaling, photosynthesis and systemic acquired acclimation. Front Physiol. 2017;8:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007;30(3):249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 46.Yan X, et al. Research progress on electrical signals in higher plants. Progress Nat Sci. 2009;19(5):531–541. [Google Scholar]
- 47.Volkov A, et al. Circadian variations in biologically closed electrochemical circuits in Aloe vera and. Mimosa Pudica Bioelectrochemistry. 2011b;81(1):39–45. [DOI] [PubMed] [Google Scholar]
- 48.Volkov A, Shtessel Y. Electrotonic signal transduction between aloe vera plants using underground pathways in soil: experimental and analytical study. AIMS Biophysics. 2017;4:576. [Google Scholar]
- 49.Volkov A, Shtessel Y. Electrical signal propagation within and between tomato plants. Bioelectrochemistry. 2018;124:195–205. [DOI] [PubMed] [Google Scholar]
- 50.Awan H, et al. Communication in plants: comparison of multiple action potential and mechanosensitive signals with experiments. IEEE Trans Nanobioscience. 2020;19(2):213–223. doi: 10.1109/TNB.2019.2951289. [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee S (2017). An Approach Towards Plant Electrical Signal Based External Stimuli Monitoring System. Ph.D. thesis, University of Southampton. [Google Scholar]
- 52.Chatterjee S, Malik O, Gupta S. Chemical sensing employing plant electrical signal response-classification of stimuli using curve fitting coefficients as features. Biosensors. 2018;8(3):1–21. doi: 10.3390/bios8030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hope A. The action potential in cells of chara. Nature. 1961;191:811–812. [Google Scholar]
- 54.Mullins LJ. Efflux of chloride ions during the action potential of nitella. Nature. 1962;196:986–987. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, et al. Ca2+ channels and signals involved in abiotic stress responses in plant cells: recent advances. Plant Cell, Tissue and Organ Culture (PCTOC). 2018;132(3):413–424. [Google Scholar]
- 56.Hedrich R. 1994. Voltage-dependent chloride channels in plant cells: identification, characterization, and regulation of a guard cell anion channel. Chloride Channels. Vol. 42. 1–33. Academic Press. [Google Scholar]
- 57.Cuin TA, Dreyer I, Michard E. The role of potassium channels in arabidopsis thaliana long distance electrical signalling: AKT2 modulates tissue excitability while GORK shapes action potentials. Int J Mol Sci. 2018;19(4):926. doi: 10.3390/ijms19040926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumari A, et al. Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proc Nat Acad Sci. 2019;116(40):20226–20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Bel A, et al. Spread the news: systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J Exp Bot. 2014;65(7):1761–1787. [DOI] [PubMed] [Google Scholar]
- 60.Zhao D, et al. High-resolution non-contact measurement of the electrical activity of plants in situ using optical recording. Sci Rep. 2015;5:13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ping Z, Mimura T, Tazawa M. Jumping Transmission of Action Potential between Separately Placed Internodal Cells of Chora corallina. Plant Cell Physiol. 1990;31:299–302. [Google Scholar]
- 62.Trontelj Z, et al. Magnetic detection of a single action potential in chara corallina internodal cells. Biophys J. 1994;66(5):1694–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts A (2007). Plasmodesmata, chapter Plasmodesmal Structure and Development, 1–32. Blackwell Publishing Ltd. [Google Scholar]
- 64.Choi W, Miller G, Wallace I, Harper J, Mittler R, Gilroy S. Orchestrating rapid long-distance signaling in plants with Ca 2+++, ROS and electrical signals. Plant J. 2017;90(4):698–707. doi: 10.1111/tpj.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sukhov V, et al. Simulation of action potential propagation in plants. J Theor Biol. 2011;291:47–55. [DOI] [PubMed] [Google Scholar]
- 66.Kurenda A, et al. Insect-damaged Arabidopsis moves like wounded. Mimosa Pudica Proc Nat Acad Sci. 2019;116(51):26066–26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen CT, et al. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc Nat Acad Sci. 2018;115(40):10178–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monshausen G, Haswell E. A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot. 2013;64:4663–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iosip A, et al. The venus flytrap trigger hair–specific potassium channel KDM1 can reestablish the K+ gradient required for hapto-electric signaling. PLoS Biol. 2020;18(12):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meena M, et al. The Ca2+ channel CNGC19 regulates arabidopsis defense against spodoptera herbivory. Plant Cell. 2019;31(7):1539–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thor K, et al. The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature. 2020;(585):569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tian W, et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature. 2019;(572):131–135. [DOI] [PubMed] [Google Scholar]
- 73.Khlopkov A, et al. Participation of calcium ions in induction of respiratory response caused by variation potential in pea seedlings. Plant Signal Behav. 2021;16(4):1869415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans N, McAinsh MR, Hetherington AM. Calcium oscillations in higher plants. Curr Opin Plant Biol. 2001;4(5):415–420. doi: 10.1016/S1369-5266(00)00194-1. [DOI] [PubMed] [Google Scholar]
- 75.Kudla J, et al. Advances and current challenges in calcium signaling. New Phytologist. 2018;218(2):414–431. [DOI] [PubMed] [Google Scholar]
- 76.Sukhova E, Akinchits E, Sukhov V. Mathematical models of electrical activity in plants. J Membr Biol. 2017;250:407–423. [DOI] [PubMed] [Google Scholar]
- 77.Mousavi S, et al. Glutamate receptor-like genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. [DOI] [PubMed] [Google Scholar]
- 78.Ramesh SA, et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6(7879):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu X, et al. Signaling role of glutamate in plants. Front Plant Sci. 2020;10:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toyota M, et al. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018;361(6407):1112–1115. [DOI] [PubMed] [Google Scholar]
- 81.Lapeikaite I, et al. Glutamate and NMDA affect cell excitability and action potential dynamics of single cell of macrophyte nitellopsis obtusa. Functional Plant Biology. 2020;47(12):1032–1040. [DOI] [PubMed] [Google Scholar]
- 82.Ramesh S, et al. γ-aminobutyric acid (GABA) signalling in plants. Cellular Molecul Life Sci Volume. 2017;74(9):1577–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouché N, Fromm H. GABA in plants: just a metabolite? Trends Plant Sci. 2004;9(3):110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Kinnersley AM, Turano FJ. Gamma Aminobutyric Acid (GABA) and plant responses to stress. CRC Crit Rev Plant Sci. 2000;19:479–509. [Google Scholar]
- 85.De Luccia T. Mimosa pudica, Dionaea muscipula and anesthetics. Plant Signal Behav. 2012;7(9):1163–1167. doi: 10.4161/psb.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses in. Mimosa Pudica Canad J Anesthesia. 1999;46:287–289. [DOI] [PubMed] [Google Scholar]
- 87.Saltveit M. Effect of high-pressure gas atmospheres and anaesthetics on chilling injury of plants. J Exp Bot. 1993;44:1361–1368. [Google Scholar]
- 88.Weigl J. Membrane structure in plants: effect of xenon on the Mimosa reaction. Zeitschrift Fur Naturforschung Teil B, Chemie, Biochemie, Biophysik, Biologie Und Verwandte Gebiete. 1968;23:9. [PubMed] [Google Scholar]
- 89.Gall S, et al. Anaesthetics stop diverse plant organ movements, affect endocytic vesicle recycling and ROS homeostasis, and block action potentials in Venus flytraps. Ann Bot. 2018;122(5):747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shepherd V, et al. Mechanosensory ion channels in charophyte cells: the response to touch and salinity stress. Eur Biophys J. 2002;(31):341–355. [DOI] [PubMed] [Google Scholar]
- 91.Baluška F, et al. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci. 2005;10(3):3. doi: 10.1016/j.tplants.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Baluška F, Schlicht M, Volkmann D, Mancuso S. Vesicular secretion of auxin. Plant Signal Behav. 2008;3(4):254–256. doi: 10.4161/psb.3.4.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mettbach U, et al. Immunogold-EM analysis reveal brefeldin a-sensitive clusters of auxin in arabidopsis root apex cells. Commun Integr Biol. 2017;10(3):e1327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlicht M, et al. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav. 2006;1(3):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hertel R. Vesicles accumulating auxin in vitro are not “presynaptic”. Plant Physiol. 2018;176:1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robinson DG, et al. Auxin and vesicle traffic. Plant Physiol. 2018;176(3):1884–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baluška F, Strnad M, Mancuso S. Substantial evidence for auxin secretory vesicles. Plant Physiol. 2018;176(4):2586–2587. doi: 10.1104/pp.18.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baluška F, Mancuso S (2009). Plant-Environment Interactions: from Sensory Plant Biology to Active Plant Behavior, chapter Plants and Animals: Convergent Evolution in Action?, 285–301. Springer Berlin Heidelberg. [Google Scholar]
- 99.Geisler M. Seeing is better than believing: visualization of membrane transport in plants. Curr Opin Plant Biol. 2018;46:104–112. [DOI] [PubMed] [Google Scholar]
- 100.Baluška F, Mancuso S. Root apex transition zone as oscillatory zone. Front Plant Sci. 2013;4:354. doi: 10.3389/fpls.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yokawa K, et al. Binary decisions in maize root behavior: y-maze system as tool for unconventional computation in plants. Plant Signal Behav. 2014;10(5–6):381–390. [Google Scholar]
- 102.Masi E, et al. The electrical network of maize root apex is gravity dependent. Sci Rep. 2015;(5):7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gagliano M, et al. Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia. 2014;175(1):63–72. [DOI] [PubMed] [Google Scholar]
- 104.Gagliano M, et al. Plants learn and remember: lets get used to it. Oecologia. 2018;186:1. [DOI] [PubMed] [Google Scholar]
- 105.Biegler R. Insufficient evidence for habituation in Mimosa pudica. response to gagliano et al. (2014). Oecologia. 2018;186(1):33–35. doi: 10.1007/s00442-017-4012-3. [DOI] [PubMed] [Google Scholar]
- 106.Kelly C. Resource choice in cuscuta europaea. Proc Nat Acad Sci. 1992;89:12194–12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koch A, et al. Does the generalist parasitic plant cuscuta campestris selectively forage in heterogeneous plant communities? New Phytologist. 2004;162(1):147–155. [Google Scholar]
- 108.Furuhashi T, Furuhashi K, Weckwerth W. The parasitic mechanism of the holostemparasitic plant Cuscuta. J Plant Interact. 2011;6(4):207–219. doi: 10.1080/17429145.2010.541945. [DOI] [Google Scholar]
- 109.HarperCollins . Nervous system. In: Collins English Dictionary. HarperCollins; 2012. [Google Scholar]
- 110.Cambridge University Press (n.d.) . Nervous system. In: Cambridge Advanced Learner’s Dictionary. Cambridge University Press; 2013. [Google Scholar]
- 111.Merriam-Webster. (n.d .) (2021). Nervous system. In Merriam-Webster.com dictionary. Merriam-Webster.[cited on 21 May 2021] https://www.merriam-webster.com/dictionary/nervous%20system [Google Scholar]
- 112.Houghton Mifflin Harcourt Publishing Company (n.d.) . Nervous system. In: The American Heritage Science Dictionary. Houghton Mifflin Harcourt Publishing Company; 2011. [Google Scholar]
- 113.Bear M, et al. Neuroscience: exploring the Brain. 3rd edition ed. Baltimore: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 114.Moroz L. On the independent origins of complex brains and neurons. Brain Behav Evolution. 2009;74:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moroz L, et al. The ctenophore genome and the evolutionary origins of neural systems. Nature. 2014;(510):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moroz L, Kohn A. Independent origins of neurons and synapses: insights from ctenophores. Philosophical Transac Royal Soc London B: Biol Sci. 2016;371:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arendt D, et al. From nerve net to nerve ring, nerve cord and brain — evolution of the nervous system. Nat Rev Neuroscice. 2016;(17):61–72. doi: 10.1038/nrn.2015.15. [DOI] [PubMed] [Google Scholar]
- 118.Miller G. On the origin of the nervous system. Science. 2009;325:24–26. [DOI] [PubMed] [Google Scholar]
- 119.Varoqueaux F, Fasshauer D. Getting nervous: an evolutionary overhaul for communication. Annu Rev Genet. 2017;51:455–476. [DOI] [PubMed] [Google Scholar]
- 120.Hsia CCW, Schmitz A, Lambertz M, Perry SF, Maina JN (2013). Comprehensive Physiology, chapter Evolution of Air Breathing: Oxygen Homeostasis and the Transitions from Water to Land and Sky, 849–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Woods H, Smith J. Universal model for water costs of gas exchange by animals and plants. Proc Nat Acad Sci. 2010;107:8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mesarovic M, Takahara Y. General Systems Theory: mathematical Foundations. Academic Press, New York; 1975. [Google Scholar]
- 123.Von Bertalanffy L. General System Theory: foundations, Development, Applications, sixteenth ed. George Braziller; 1969. [Google Scholar]
- 124.Hammond C. Cellular and Molecular Neurobiology, 2nd ed. Academic Press; 2001. [Google Scholar]
- 125.Llinás R. Commentary on “electrophysiological properties of in vitro purkinje cell dendrites in mammalian cerebellar slices. j physiol 1980;305:197–213. Cerebellum. 2012;11:629. [DOI] [PubMed] [Google Scholar]
- 126.Llinás R, Sugimori M. Electrophysiological properties of in vitro purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suzuki M, Larkum M. Dendritic calcium spikes are clearly detectable at the cortical surface. Nat Commun. 2017;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Llinás R, et al. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Nat Acad Sci. 1989;86(5):1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fromm J, Spanswick R. Characteristics of Action Potentials in Willow (Salix viminalis L.). J Exp Bot. 1993;44(7):1119–1125. doi: 10.1093/jxb/44.7.1119. [DOI] [Google Scholar]
- 131.Thuleau P, et al. Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J. 1994;13(13):2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baudenbacher F, Fong LE, Thiel G, Wacke M, Jazbinsek V, Holzer JR, Stampfl A, Trontelj Z. Intracellular axial current in chara corallina reflects the altered kinetics of ions in cytoplasm under the influence of light. Biophys J. 2005;88(1):690–697. doi: 10.1529/biophysj.104.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382(6593):719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- 134.Koselski M, et al. Impact of mammalian two-pore channel inhibitors on long-distance electrical signals in the Characean Macroalga Nitellopsis obtusa and the early terrestrial liverwort. Marchantia Polymorpha Plants. 2021;10(4):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. [DOI] [PubMed] [Google Scholar]
- 136.Straub V, et al. Endogenous and network properties of lymnaeafeeding central pattern generator interneurons. J Neurophysiol. 2002;88(4):1569–1583. [DOI] [PubMed] [Google Scholar]
- 137.Watanabe A, et al. Pacemaker potentials for the periodic burst discharge in the heart ganglion of a stomatopod, squilla oratoria. J General Physiol. 1967;50(4):839–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fain G, O’Dell T. Molecular and Cellular Physiology of Neurons, Second Edition, 2nd ed. Harvard University Press; 2014. [Google Scholar]
- 139.Bach-y Rita P. Nonsynaptic diffusion neurotransmission in the brain: functional considerations. Neurochem Res. 2001;26(8/9):871–873. doi: 10.1023/A:1012300914150. [DOI] [PubMed] [Google Scholar]
- 140.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Zhang W-B, Agnati LF. Volume transmission and its different forms in the central nervous system. Chin J Integr Med. 2013;19(5):323–329. doi: 10.1007/s11655-013-1455-1. [DOI] [PubMed] [Google Scholar]
- 141.Baker R, Llinás R. Electrotonic coupling between neurones in the rat mesencephalic nucleus. J Physiol. 1971;212(1):45–63. doi: 10.1113/jphysiol.1971.sp009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baluška F, Mancuso S. Plant neurobiology: from sensory biology, via plant communication, to social plant behavior. Cogn Process. 2009;10(S1):3–7. doi: 10.1007/s10339-008-0239-6. [DOI] [PubMed] [Google Scholar]
- 143.Llinás R. The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2014;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spanswick RM. Electrical coupling between cells of higher plants: a direct demonstration of intercellular communication. Planta. 1972;102:215–227. [DOI] [PubMed] [Google Scholar]
- 145.Cortes PM. Cortical intracellular electrical potential in roots of unstressed and stressed sunflower seedlings. ii. radial profiles and oscillations. Functional Plant Biology. 1997;24(5):651–660. doi: 10.1071/PP96037. [DOI] [Google Scholar]
- 146.Volkov A, et al. Electrotonic and action potentials in the venus flytrap. J Plant Physiol. 2013a;170(9):838–846. [DOI] [PubMed] [Google Scholar]
- 147.Volkov A, Shtessel Y. Propagation of electrotonic potentials in plants: experimental study and mathematical modeling. AIMS Biophysics. 2016;3:358–379. [Google Scholar]
- 148.Iijima T, Sibaoka T. Propagation of action potential over the trap-lobes of Aldrovanda vesiculosa. Plant Cell Physiol. 1982;23:679–688. [Google Scholar]
- 149.Masi E, et al. Resting electrical network activity in traps of the aquatic carnivorous plants of the genera aldrovanda and utricularia. Sci Rep. 2016;6(24989):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gibbons C. Chapter 27 - basics of autonomic nervous system function. In: Clinical Neurophysiology: basis and Technical Aspects, volume 160 of Handbook of Clinical Neurology. Elsevier; 2019. p. 407–418. [DOI] [PubMed] [Google Scholar]
- 151.Fromm J, Hajirezaei M, Wilke I. The biochemical response of electrical signaling in the reproductive system of hibiscus plants. Plant Physiol. 1995;109(2):375–384. doi: 10.1104/pp.109.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Farmer E, Gao Y-Q, Lenzoni G, Wolfender J-L, Wu Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytologist. 2020;227(4):1037–1050. doi: 10.1111/nph.16646. [DOI] [PubMed] [Google Scholar]
- 153.Wang J, et al. Jasmonate action in plant defense against insects. J Exp Bot. 2019;70(13):3391–3400. [DOI] [PubMed] [Google Scholar]
- 154.Ulrich-Lai Y, Herman J. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscice. 2009;10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Darwin C. Insectivorous plants. London: John Murray; 1875. [Google Scholar]
- 156.Darwin C. The movements and habits of climbing plants. London: John Murray; 1875. [Google Scholar]
- 157.Darwin C. The power of movement in plants. London: John Murray; 1880. [Google Scholar]
- 158.Darwin C. Origin of Species. London: John Murray; 1859. [Google Scholar]
- 159.Llinás R. The contribution of Santiago Ramón y Cajal to functional neuroscience. Nat Rev Neuroscice. 2003;4:77–80. [DOI] [PubMed] [Google Scholar]
- 160.Bullock T, Bennett MVL, Johnston D, Josephson R, Marder E, Fields RD. The neuron doctrine, redux. Science. 2005;310(5749):791–793. doi: 10.1126/science.1114394. [DOI] [PubMed] [Google Scholar]
- 161.Juusola M, et al. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19(7):292–297. [DOI] [PubMed] [Google Scholar]
- 162.Wilson M. Synaptic physiology: plenty of models to choose from. Curr Biol. 2004;14:R666–R667. [DOI] [PubMed] [Google Scholar]
- 163.Zoli M, Agnati L. Wiring and volume transmission in the central nervous system: the concept of closed and open synapses. Prog Neurobiol. 1996;49:363–380. [DOI] [PubMed] [Google Scholar]
- 164.Ramón Y Cajal S. La Textura del Sistema Nervioso del Hombre y Los Vertebrados. Imprenta de Nicolás Moya; 1904. Vol. 3. Madrid. [Google Scholar]
- 165.Ramón Y Cajal S. ¿Neuronismo o reticularismo?: las pruebas objetivas de la unidad anatómica de las células nerviosas. Archivos de Neurobiología. 1933;13::271–291,579–646. [Google Scholar]
- 166.Shubin N, et al. Deep homology and the origins of evolutionary novelty. Nature. 2009;(457):818–823. [DOI] [PubMed] [Google Scholar]
- 167.Zakon HH. Adaptive evolution of voltage-gated sodium channels: the first 800 million years. Proc Nat Acad Sci. 2012;109:10619–10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Edel K, Marchadier E, Brownlee C, Kudla J, Hetherington AM. The evolution of calcium-based signalling in plants. Curr Biol. 2017;27(13):667–679. doi: 10.1016/j.cub.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 169.Chiu J, DeSalle R, Lam HM, Meisel L, Coruzzi G. Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged. Mol Biol Evol. 1999;16(6):826–838. doi: 10.1093/oxfordjournals.molbev.a026167. [DOI] [PubMed] [Google Scholar]
- 170.Qi Z, et al. Calcium Entry Mediated by GLR3.3, an Arabidopsis Glutamate Receptor with a Broad Agonist Profile. Plant Physiol. 2006;142(3):963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tapken D, et al. A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids. Sci Signal. 2013;6(279):ra47. [DOI] [PubMed] [Google Scholar]
- 172.Lynagh T, others . Molecular basis for convergent evolution of glutamate recognition by pentameric ligand-gated ion channels. Sci Report. 2015;5:8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Žárský V. Signal transduction: gaba receptor found in plants. Nat Plants. 2015;1:15115. [DOI] [PubMed] [Google Scholar]
- 174.Turano F, et al. The putative glutamate receptors from plants are related to two superfamilies of animal neurotransmitter receptors via distinct evolutionary mechanisms. Mol Biol Evol. 2001;18(7):1417–1420. [DOI] [PubMed] [Google Scholar]
- 175.Bishop G. Natural history of the nerve impulse. Physiol Rev. 1956;36(3):376–399. doi: 10.1152/physrev.1956.36.3.376. [DOI] [PubMed] [Google Scholar]
- 176.Liebeskind B, et al. Evolution of animal neural systems. Annu Rev Ecol Evol Syst. 2017;48(1):377–398. [Google Scholar]
- 177.Barlow P. Reflections on ‘plant neurobiology’. Biosystems. 2008;92(2):132–147. doi: 10.1016/j.biosystems.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 178.Schwann T. Mikroskopische Untersuchungen über die Uebereinstimmung in der Struktur und dem Wachsthum der Thiere und Pflanzen. G.E. Reimer; 1839. Berlin. [PubMed] [Google Scholar]
- 179.Shannon C. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. [Google Scholar]
- 180.François P, Altan-Bonnet G. The case for absolute ligand discrimination: modeling information processing and decision by immune T cells. J Stat Phys. 2016;162(5):1130–1152. doi: 10.1007/s10955-015-1444-1. [DOI] [Google Scholar]
- 181.Fromm H, Dietz K-J. GABA signaling in plants: targeting the missing pieces of the puzzle. J Exp Bot. 2020;71(20):6238–6245. doi: 10.1093/jxb/eraa358. [DOI] [PubMed] [Google Scholar]
- 182.Flis A, Mengin V, Ivakov AA, Mugford ST, Hubberten H-M, Encke B, Krohn N, Höhne M, Feil R, Hoefgen R, et al. Multiple circadian clock outputs regulate diel turnover of carbon and nitrogen reserves. Plant Cell Environ. 2019;42(2):549–573. doi: 10.1111/pce.13440. [DOI] [PubMed] [Google Scholar]
- 183.Scialdone A, et al. Arabidopsis plants perform arithmetic division to prevent starvation at night. Elife. 2013;2:e00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Band L, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peret B, et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Nat Acad Sci. 2012;109(12):4668–4673. doi: 10.1073/pnas.1201498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee Z, et al. The roles of plant hormones and their interactions with regulatory genes in determining meristem activity. Int J Mol Sci. 2019;20(16):4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Leopold A, Noodén L (1984). Hormonal Regulation of Development II: the Functions of Hormones from the Level of the Cell to the Whole Plant, chapter Hormonal Regulatory Systems in Plants, 4–22. Springer Berlin Heidelberg. [Google Scholar]
- 187.Llinás R, Iberall A. A global model of neuronal command-control systems. Biosystems. 1977;8:233–235. [DOI] [PubMed] [Google Scholar]
- 188.Djouhri L, Lawson S. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev. 2004;46(2):131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 189.Zalc B. Purinergic Signalling in Neuron–Glia Interactions, chapter The Acquisition of Myelin: a Success Story, pages 15–25. UK: Ltd: John Wiley & Sons; 2006. [Google Scholar]
- 190.Seidl A. Regulation of conduction time along axons. Neuroscience. 2014;276:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sugihara I, Lang E, Llinás R. Uniform olivocerebellar conduction time underlies purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Porras A, Llinás R. Bio-inspired coupled oscillatory phase reset control system applied to movement in an underwater vehicle. Rob Auton Syst. 2014;62:257–266. [Google Scholar]
- 193.Mayr E, Bock W. Classifications and other ordering systems. J Zool Systemat Evol Res. 2002;40:169–194. [Google Scholar]
- 194.Miguel-Tomé S. The influence of computational traits on the natural selection of the nervous system. Nat Comput. 2018;17:403–425. [Google Scholar]
- 195.Sipser M. Introduction to the Theory of Computation, 3rd ed. Boston: Cengage Learning; 2012. [Google Scholar]
- 196.Lichtenthaler H. The stress concept in plants: an introduction. Ann N Y Acad Sci. 1998;851:187–198. [DOI] [PubMed] [Google Scholar]
- 197.Banker G. The development of neuronal polarity: a retrospective view. J Neurosci. 2018;38(8):1867–1873. doi: 10.1523/JNEUROSCI.1372-16.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Llinás R. The Squid Giant Synapse: a Model for Chemical Transmission. Oxford University Press, Oxford; 1999. [Google Scholar]
- 199.Nicoll R. A brief history of long-term potentiation. Neuron. 2017;93:281–290. [DOI] [PubMed] [Google Scholar]
- 200.Moroz L, et al. Neural versus alternative integrative systems: molecular insights into origins of neurotransmitters. Philosophical Transac Royal Soc London B: Biol Sci. 2021;376(1821):20190762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ryan T, Grant S. The origin and evolution of synapses. Nat Rev Neuroscice. 2009;10:701–712. [DOI] [PubMed] [Google Scholar]
- 202.Mackie GO. Neuroid conduction and the evolution of conducting tissues. Q Rev Biol. 1970;45:319–332. [DOI] [PubMed] [Google Scholar]
- 203.Mackie GO. The elementary nervous system revisited. Am Zool. 1990;30:907–920. [Google Scholar]
- 204.Parker G. -The Elementary Nervous Systems. . J. B. Lippincott Company, Philadelphia; 1919. [Google Scholar]
- 205.De Wiljes O, Van Elburg RAJ, Keijzer FA. Modelling the effects of short and random proto-neural elongations. Journal of the Royal Society Interface. 2017;14(135):20170399. doi: 10.1098/rsif.2017.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jékely G. Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc Royal Soc London B: Biol Sci. 2011;278:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rasband M. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. [DOI] [PubMed] [Google Scholar]
- 208.Borroto-Escuela D, Agnati LF, Bechter K, Jansson A, Tarakanov AO, Fuxe K. The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural–glial networks. Philosophical Transac Royal Soc London B: Biol Sci. 2015;370(1672):20140183. doi: 10.1098/rstb.2014.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ovsepian S. The birth of the synapse. Brain Struct Funct. 2017;222:3369–3374. [DOI] [PubMed] [Google Scholar]
- 210.Cherniak C. Component placement optimization in the brain. J Neurosci. 1994;14(4):2418–2427. doi: 10.1523/JNEUROSCI.14-04-02418.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cobb J (1995). The Nervous Systems of Invertebrates: an Evolutionary and Comparative Approach, chapter The nervous systems of Echinodermata: Recent results and new approaches, 407–424. Birkhäuser Basel. [Google Scholar]
- 212.Cobb JLS (1987). The Nervous Systems of Invertebrates, chapter Neurobiology of the Echinodermata, 483–525. Springer US. [Google Scholar]
- 213.Volkov AG. Biosensors, memristors and actuators in electrical networks of plants. Int J Parallel, Emergent Distributed Systems. 2017;32:44–55. [Google Scholar]
- 214.Hedrich R, Salvador-Recatalá V, Dreyer I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 2016;21:376–387. [DOI] [PubMed] [Google Scholar]
- 215.Morrison R, Boyd R. Organic Chemistry, 6 ed. Prentice Hall, New Jersey; 1992. [Google Scholar]
- 216.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973;35(3):125–129. doi: 10.2307/4444260. [DOI] [Google Scholar]


