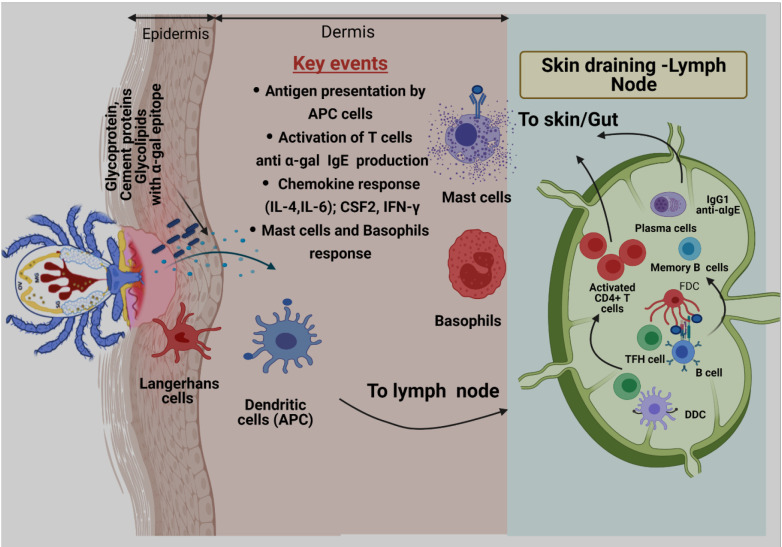
Figure 2.
Proposed model of α-gal sensitization from tick bites. Skin is comprised of three layers: epidermis, dermis, and hypodermis. Antigen-presenting cells (APCs), including Langerhans cells (LCs) and dermal Dendritic cells (DCs) residing in epidermis and dermis, respectively, respond to tick-secreted antigens, such as glycoproteins, glycolipids, and tick cement-containing α-gal moieties. After antigen exposure, APCs process antigen, migrate to skin-draining lymph nodes, and participate in allergen sensitization. During this process, naïve T cells are primed through presentation of tick α-gal antigens by LCs and dermal DCs within skin-draining lymph nodes. Activated CD4+ T cells subsequently traffic to the skin through blood and lymphatic vessels. Cognate T cell help, provided by T follicular helper (TFH) cells, to α-gal-specific B cells leads to germinal center responses, positive clonal selection of B cells via recognition of native antigens retained by follicular dendritic cells (FDCs), and the development of memory B cells and plasma cells. After clonal selection, B cells migrate to the tick bite site on the skin to manifest allergic responses by presenting antigens to T cells, secreting proinflammatory cytokines, and secreting α-gal-specific antibodies (anti-α-IgE) that ultimately triggers activation of mast cells and basophils and allergic response.