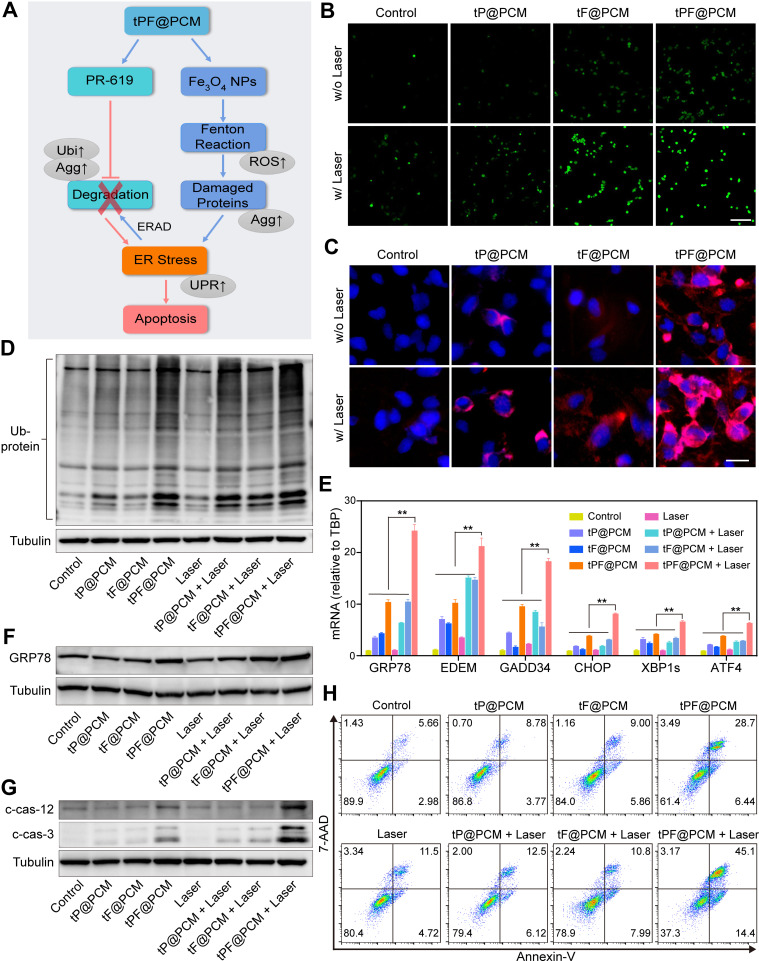
Figure 4.
Exacerbating ER stress by tPF@PCM with laser irradiation induces apoptosis in 786-O cells. (A) Schematic overview of the mechanism of synergistic apoptosis. Fe3O4 NPs-triggered Fenton reaction led to increased damaged proteins in the ER lumen and caused initial ER stress. ERAD pathway was then activated to degrade these proteins (blue arrows). However, PR-619 inhibited the degradation of the stacked damaged proteins, which further exacerbated the existing ER stress. Ultimately, unsalvageable ER stress induced apoptosis in 786-O RCC cells (red arrows). The grey ellipses represent the corresponding intracellular biological changes. (B) Confocal laser scanning microscopy images of intracellular ROS detection in 786-O cells from different groups. Scale bar represents 200 μm. (C) Fluorescence images of protein aggregates in 786-O cells after various treatments. Cell nuclei were stained with Hoechst 33342 (blue) and protein aggregates were stained with PROTEOSTAT dye (red). Scale bar represents 25 μm. (D) Western blot analysis of total ubiquitinated proteins in 786-O cells after different treatments. Tubulin was used as a loading control. (E) mRNA expression of UPR target genes in 786-O cells after different treatments. TBP mRNA levels were used as internal controls. **P < 0.01. (F–G) Western blot analysis of GRP78 (F), cleaved caspase-12 (c-cas-12), and cleaved caspase-3 (c-cas-3) (G) expression in 786-O cells after different treatments. Tubulin was used as a loading control. (H) Apoptosis detection of 786-O cells after various treatments using annexin V-PE/7-AAD kit by flow cytometry. Early apoptotic cells were analyzed as annexin V+/7-AAD−, whereas late apoptotic/necrotic cells were determined as annexin V+/7-AAD+.
Abbreviations: ROS, reactive oxygen species; Agg, aggresome; UPR, unfolded protein response; Ubi, ubiquitination.