Abstract
Purpose of Review
Heart centers for women (HCW) were developed due to the rising cardiovascular morbidity and mortality in women in the United States in the early 1990s. Our review encompasses the epidemiology, risk factors, diagnostic strategies, treatments, and the role of HCW in managing women with ischemic heart disease (IHD).
Recent Findings
HCW use a multidisciplinary team to manage women with IHD. Due to the paucity of randomized controlled trials investigating various manifestations of IHD, some treatments are not evidence-based such as those for coronary microvascular dysfunction and spontaneous coronary artery dissection. Sex-specific risk factors have been identified and multimodality cardiac imaging is improving in diagnosing IHD in women. Treatments are being studied to help improve symptoms and outcomes in women with IHD.
Summary
There has been progress in the care of women with IHD. HCW can be instrumental in treating women with IHD, doing research, and being a source of research study participants.
Keywords: Myocardial infarction with non-obstructive coronary arteries (MINOCA), Ischemia with non-obstructive coronary arteries (INOCA), Multidisciplinary healthcare delivery, Sex differences in cardiovascular disease, Non-obstructive coronary artery disease
Introduction
Cardiovascular disease (CVD) remains the number one cause of mortality in women mostly due to ischemic heart disease (IHD) and stroke and appears to be increasing globally [1••]. The burden of CVD has continued to increase for decades in almost all countries except for the high-income countries [1••]. Of great concern is trend of rising mortality from CVD in women and men in high-income countries such as the United States (US), where it was declining previously from 2000 to 2010 in women [2••]. This rising trend of CVD mortality in women was also seen in the 1990s when heart disease in women was less understood. In the 1980s and 1990s, the prevention of IHD in women differed from men due partly to the observational findings of the Nurses’ Health Study (NHS) in 1985. The use of HRT was associated with a 50% reduction in the risk of coronary artery disease (CAD) outcomes in the 5-year follow-up NHS in 1985 [3]. In the same year, the Framingham Heart Study showed a 50% increased risk of cardiovascular morbidity and doubled the risk of cerebrovascular disease [4••] in women using HRT compared to those who were not. Ultimately, NHS confirmed similar results in their 10-year follow-up study [5] and many postmenopausal women continued to be treated with HRT.
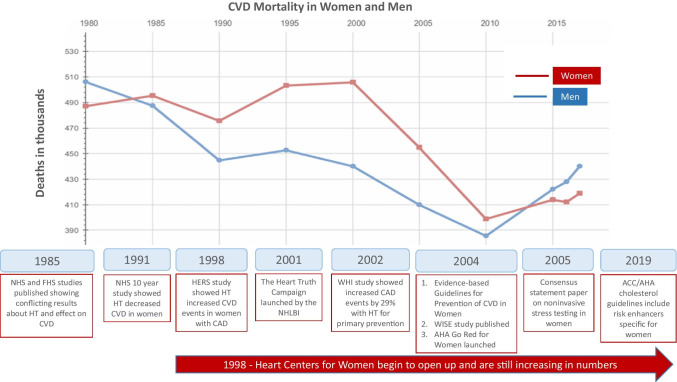
In the 1990s, cardiovascular mortality increased in women while decreasing in men [2••]. In the late 1990s and early 2000s, several randomized controlled trials (RCTs) revealed that HRT did not prevent CVD [6, 7]. The observational studies may have led to the increased CVD mortality seen in the 1990s and galvanized the need for better research and management of women with heart disease and led to the opening of heart centers for women (HCW) [8••] and national healthcare organizations collaborated to improve cardiac care in women [9]. In 2004, sex-specific CVD guidelines emphasized the harm of HRT to prevent CVD and strongly encouraged the use of evidence-based therapies to lower CVD risk in women [10]. Guideline-derived medical therapy (GDMT), including diagnostic testing, lifestyle management, and treatments, was provided to women [8••]. All these efforts are associated with the decline of CVD mortality in women from its peak in 2000 to its nadir in 2010 (Fig. 1).
Fig. 1.
Timeline of mortality in women and men from cardiovascular disease from 1980 to 2017. Timeline of observational (NHS, FHS, WISE) and randomized placebo-controlled studies (HERS and WHI) that led to guidelines and the management of ischemic heart disease in women. Also shown are the initiation of national campaigns to increase awareness of heart disease in women (NHLBI Heart Truth campaign and AHA Go Red for Women) and heart centers for women in the United States. Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; CAD, coronary artery disease; CVD, cardiovascular disease; FHS, Framingham Heart Study; HERS, Heart and Estrogen/progestin Replacement Study; NHLBI, National Heart Lung and Blood Institute; NHS, Nurses’ Health Study; WISE, Women’s Ischemia Syndrome Evaluation. (Reprinted with permission Heart Disease and Stroke Statistics – Update 2021 ©2021 American Heart Association, Inc.) [4••]
Figure 1 shows the timeline of events that led to the current management of IHD in women. CVD mortality in both men and women is increasing since 2010, which is alarming and needs to be addressed. This review will summarize the management of IHD in women and the role of HCW in these patients’ care.
Epidemiology of CAD in Women
CVD, comprised of CAD, heart failure (HF), stroke, and hypertension, is the leading cause of death of women in the US and globally. In American women, a prevalence of 48.4% resulted in 420,164 deaths in 2018, mostly due to CAD. The overall number of CVD deaths is slightly higher in men. However, sub-analysis by ethnicity shows that women account for a higher percentage of deaths in Blacks and Hispanics [2••]. Despite a downtrend in CVD mortality for both men and women in the US from 2000 to 2010, rates have been increasing since 2010 for both sexes, [11••] particularly notable in women age 35–54 years [2••, 12].
Women experience their first myocardial infarction (MI) roughly 10 years later than men, typically after menopause [13]. They are more likely to have longer door-to-balloon times, a lower rate of prescribed GDMT, and higher in-hospital mortality [2••]. Even after a successful percutaneous coronary intervention (PCI), women with acute coronary syndrome (ACS) have a 20% higher adjusted mortality risk in the short term, and at 1 year for those over age 45, a 74% survival rate vs. 81% in men [14•]. For those who survive an ACS, there is a higher risk of recurrent MI, HF, and death [15]. This risk is striking in women under 50 years old, where there is twice-higher mortality than men [16]. Overall, short- and long-term mortality with IHD is 40% higher in women after adjusting for age and comorbidities [12]. Given these statistics, all clinicians can significantly mitigate risk factors in women to decrease CAD development and IHD.
Definition and Risk Factors for IHD
IHD can be categorized as an acute or chronic condition. IHD can be due to obstructive and non-obstructive (defined as < 50% stenosis in epicardial coronary arteries) CAD. Traditional risk factors including hypertension, hyperlipidemia, diabetes, smoking, sedentary lifestyle, family history, chronic kidney disease, peripheral artery disease, and obesity are potentially modifiable contributors [17••]. More recent data investigating adverse pregnancy outcomes, systemic inflammatory disorders, obstructive sleep apnea, cancer-based therapy, and psychosocial contributors may disproportionately affect women [18•]. In implementing a HCW, it is critical to have relationships with maternal fetal medicine, obstetrics, specialists in cardio-oncology, behavioral psychologists, and social work among many other disciplines to ensure adequate risk factor identification and treatment [8••]. Barriers to heart care for women can be the unavailability of these services for patients at the medical facility.
Multiple reports indicate lower socioeconomic status (SES) in both sexes is associated with increased MI and death rates than those of higher SES, and it is a more powerful risk factor in women than in men [19•, 20•]. Those with an annual household income of < $20,000 have a 4.91-fold higher relative risk ratio of MI and cardiovascular death than those in higher-income brackets [21]. With 26.8% of female-headed households living below the US’s poverty threshold, this subgroup is particularly susceptible [22]. Additionally, more social determinants of health (SDH) were associated with the increased age-adjusted incidence per 1000 person-years for fatal CHD [≥ 3 SDH 2.86 vs. 0 SDH 1.3] and non-fatal MI [≥ 3 SDH 5.44 vs. 3.91]. CV risk prediction calculators have begun to incorporate SES into their models [23].
Furthermore, research has demonstrated a dose effect between increasing SDHs and reduced odds of meeting clinical benchmarks for CVD risk factor control, i.e., blood pressure less than 140/90 mm Hg, A1C less than 7%, physical activity of more than 150 min per week, and not smoking [24]. Additionally, emerging evidence suggests that depression and psychosocial stressors mediate associations between SDH and cardiovascular risk, underscoring the need for inclusion of clinical health psychology on interdisciplinary teams to comprehensively mitigate cardiac risk [25]. Cardiology providers should include an SDH assessment tool in their routine patient care; develop systems for routine collection, storage, and retrieval of SDH data; develop interprofessional practice models to address SDH; and partner with community organizations to identify and support patients with SDH needs [26].
There is substantial evidence that poor mental health (e.g., depression, post-traumatic stress disorder, anxiety disorders, and other adverse psychological factors) is associated with worse cardiovascular health [27••]. The largest body of research examined the adverse bi-directional effects between depression and CAD. Depression occurs in up to one-third of patients with stable IHD, and the prevalence in women is about twice that of men [28, 29]. Depression is associated with increased risk for incident CAD, MI, and mortality following a cardiac event in both sexes and is an independent risk factor for poor prognosis in patients with ACS [27••]. Increasing evidence indicates that both direct (e.g., increased platelet aggregation, inflammation, and autonomic nervous system dysregulation) and indirect mechanisms (e.g., unhealthy lifestyle behaviors) contribute to these observed effects [27••]. HCW can address these psychosocial risks by collaborating with psychologists, psychiatrists, and social workers to facilitate urgent consultations and interventions. In a randomized, controlled trial of 237 consecutive women patients with CHD, a group-based cognitive behavioral therapy stress reduction program was associated with an almost threefold protective effect against all-cause mortality compared to usual care over the 9-year follow-up period (odds ratio, 0.33; 95% CI, 0.15 to 0.74; P = 0.007) [30].
Another randomized controlled trial included 362 women and men who experienced a CHD event in the previous 12 months and randomly assigned patients to a group-based cognitive behavioral therapy stress management program or usual care only. During the almost 8-month follow-up period, after controlling for potential confounders, patients in the intervention group had a 41% lower rate of fatal and non-fatal first recurrent CVD events (hazard ratio [95% confidence interval], 0.59 [0.42–0.83]; P = 0.002) with a strong, linear dose–response effect favoring better outcomes in those who attended more group sessions. Patients in the intervention group also experienced 45% fewer recurrent acute MIs (0.55 [0.36–0.85]; P = 0.007) [31]. Adverse pregnancy outcomes, including preterm labor, gestational diabetes, and hypertensive disorder of pregnancy, have been associated with increased risk of HF, CAD, and cardiovascular mortality by several-fold [32••, 33••]. Screening for these risk factors is critical as up to 20% of pregnancies can be affected [33••]. Cardio-obstetrics has become an essential part of HCW and requires close collaboration with obstetricians and maternal–fetal medicine specialists.
Systemic inflammatory disorders such as systemic lupus erythematosus and rheumatoid arthritis have a two- to three-fold higher risk of MI and increased CVD mortality out of proportion to traditional risk factors. Further, joint involvement may limit regular exercise, and treatment may include corticosteroids which can lead to metabolic syndrome. Collaboration with rheumatologists to study commonality in microvascular disease to facilitate treatment for these diseases’ cardiovascular manifestations is crucial [34•].
Radiation therapy can increase the rate of cardiac events by 7.4% per Gray of radiation. It is crucial to collaborate with cardio-oncology clinicians to survey and manage the cardiotoxicity of exposure to radiation, anthracyclines, monoclonal antibodies, and hormonal treatments [35].
Spontaneous coronary artery dissection (SCAD) is an increasingly recognized cause of IHD that disproportionately affects younger women and may be partly responsible for the increased mortality in 34–54-year-old women. The prevalence of typical risk factors is lower in these patients, and they account for 15–20% of acute MI during pregnancy. The risk factors for this condition are being investigated, and contributors include hormonal shifts, connective tissue disorders, fibromuscular dysplasia, myocardial bridge, and inciting triggers like intense Valsalva or stimulant use [36•].
Evaluation of IHD in Women
When evaluating women with IHD, a HCW program operates differently than some traditional prevention centers because it assesses for obstructive and non-obstructive causes for IHD.
Coronary Artery Calcium Score
Coronary artery calcium (CAC) is detected using rapid non-contrast ECG-gated CT scanning, and the CAC score can provide incremental information to refine CVD risk assessment using the Framingham risk score. The Multi-Ethnic Study of Atherosclerosis (MESA) examined the distribution of CAC in 6814 individuals with no known CAD or diabetes and followed for 10 years. The MESA calculator can reclassify individuals into the higher risk categories, identifying patients who can benefit from aggressive prevention strategies [37]. This strategy is critical in asymptomatic women who are at risk for MACE [38]. Data from the CAC consortium of 63,215 asymptomatic men and women showed similar long-term cardiovascular mortality in both men and women without CAC; however, women with CAC had a hazard ratio of 1.3 for cardiovascular mortality compared to men [39••]. These studies underscore the utility of CAC scoring in accurate CVD risk assessment and therapeutic guidance in women and is incorporated in the lipid guidelines using the pooled cohort equation [40••]. If the pooled cohort equation determination falls in the intermediate risk, the CAC score can be useful to help with the decision to treat a patient with statins without any substantial exposure to radiation [40••]. The typical radiation exposure with modern CT scanners is around 1 mSV, with newer reconstruction techniques and scanners being able to greatly minimize radiation exposure [41]. This degree of exposure is comparable to radiation from typical screening mammograms which are around 0.4 mSV and chest X-ray 0.1 mSV [42].
Stress Testing
Utilizing optimal stress tests for women is critical. Considerations in stress imaging are based on the pretest probability of IHD [43, 44•]. In women, further considerations include functional capacity, body habitus, imaging artifact, and radiation exposure to breast tissue [45]. Along with the typical presentation of angina, women may present with atypical angina symptoms [46]. Women tend to be older and have comorbidities when they become symptomatic and need evaluation for IHD, contributing to functional capacity limitations [45]. The electrocardiogram (EKG) component of exercise stress testing has a lower sensitivity and specificity for women compared to men and may lead to non-diagnostic EKG interpretation [47]. Over the last few years, there has been increasing focus on evaluating functional ischemia rather than just anatomic imaging for obstructive heart disease in women [48••].
Stress myocardial perfusion imaging (MPI) is recommended to diagnose IHD in women with intermediate to high risk who have resting EKG abnormalities [49]. MPI may be performed with single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging. SPECT is widely available and has robust data on its utility in women [49]. However, its limitations include false-positive studies from breast attenuation and obesity-related artifacts. Also, the lifetime risks related to ionizing radiation limits its use in younger women.
PET imaging has emerged over the last decade as a powerful tool in the assessment of functional ischemia, obviating the need for functional coronary angiography (FCA). It is particularly well suited for ischemic assessment in women since it has built-in attenuation correction and lowers radiation exposure than SPECT. In addition to relative perfusion, PET allows for assessing absolute quantitative coronary flow and flow reserve (CFR). CFR evaluates flow across the entire spectrum of myocardial perfusion from macrovascular to microvascular, from the epicardial arteries to the capillary level. Coronary microvascular dysfunction (CMD) is defined as functionally reduced CFR in the absence of flow-limiting CAD and is highly prevalent among at-risk individuals and is associated with adverse outcomes [50]. Impaired CFR is independently associated with cardiovascular risk and correlated with a higher non-obstructive CAD frequency in women than men [51••]. Research is needed to assess if CMD and functional ischemia are good targets for future therapeutic interventions.
CT Scan and Cardiac MRI
Coronary computed tomography angiography (CCTA) can assess many causes of IHD in one test. It has high sensitivity and diagnostic accuracy for detecting and excluding CAD and coronary anomalies in both sexes [52]. A consensus statement from the Society of Cardiovascular Computed Tomography recommends the use of CCTA for symptomatic women as an effective tool for the evaluation of CAD [53].
Multicenter trials such as Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) and Scottish Computed Tomography of the Heart (SCOT-HEART) demonstrated the ability of CCTA to improve clinical outcomes and established CCTA to be non-inferior to functional testing [54, 55]. Further, CT fractional flow reserve utilizes computational fluid dynamics and can calculate the functional significance of coronary stenosis [56]. Unlike invasive angiography, CCTA helps accurately visualize phenomena such as non-calcified, non-obstructive plaque, and external remodeling. Early detection of plaque on CCTA leads to an increase in preventive therapies [53, 57•]. Additionally, entities such as SCAD or myocardial bridging are more readily characterized on three-dimensional CCTA over invasive coronary angiography. Concerns regarding radiation exposure limited utility; however with improved technology, there has been an 80% decrease in radiation dose over the last decade [58•]. In the emergency department, early utilization of CCTA can accurately identify low-risk patients, allowing for safe, cost-effective, and expedited discharge [53, 59].
Cardiovascular magnetic resonance imaging (CMR) is a comprehensive imaging modality that can provide a detailed CVD evaluation, including myocardial structure, function, viability, ischemia, and valvular heart disease. This test is a particularly appealing option in women with suspected CMD as there is no risk of exposure to ionizing radiation with high spatial resolution and diagnostic accuracy. Stress perfusion CMR is a diagnostic modality for SD.
In the Women’s Ischemia Syndrome Evaluation (WISE) trial, which showed that women with non-obstructive coronary arteries INOCA had 2.5–3 times the risk of major adverse cardiovascular events (MACE) compared to women with normal coronary arteries [60, 61••], a myocardial perfusion reserve index of < 1.84 had a 73% sensitivity and 74% specificity to identify patients with abnormal coronary reactivity testing with a higher incidence of adverse events in those with abnormal myocardial perfusion imaging studies [62]. Given the high prevalence of myocardial infarction with non-obstructive coronary arteries (MINOCA) in women, CMR is an essential diagnostic tool that can reveal the diagnosis in up to 87% of these patients, including myocarditis and stress (Takotsubo) cardiomyopathy [63]. A recent study noted multimodality imaging (CMR and intravascular ultrasound [IVUS]) could identify the cause of MINOCA in 84.5% of women [64••].
Angiography
Among patients with angina presenting for cardiac catheterization, more than 20% have no angiographic evidence of CAD [65]. Despite this, most of these women experience persistent symptoms, recurrent hospitalizations, and poor functional status and undergo multiple catheterizations. These women have up to a three-fold increase in MACE incidence at 5 years, compared to asymptomatic controls [60, 65, 66]. Moreover, follow-up over 10 years showed cardiovascular death or MI occurs in as much as 12.8% of patients with non-obstructive disease [67]. Additional pathologic mechanisms to be considered include endothelial dysfunction, CMD, coronary spasm, oxygen-diffusion impairment, myocardial bridge, and angiographically non-evident plaques [66, 68]. In more than a third of women with MINOCA, plaque rupture or ulceration was found on IVUS [69]. Even in patients with normal angiograms, IVUS confirms the presence of atherosclerosis in the vast majority (80–100%) [64••, 69]. Positive remodeling and preserved lumen size were also common in women, limiting the utilization of angiograms [70]. Despite the absence of angiographic coronary disease, 44% had endothelial dysfunction detected by administering intracoronary acetylcholine, and 5% were found to have impaired fractional flow reserve of ≤ 0.80 through coronary physiology assessments [65].
The Coronary Microvascular Angina (CorMicA) trial provided evidence that routine management guided by an interventional diagnostic procedure and stratified therapy improves patients’ angina and quality of life [71•]. The Coronary Vasomotion Disorders International Study (COVADIS) group proposed a practical consensus approach for FCA. FCA can diagnose whether the symptoms and ischemia are due to microvascular angina, vasospastic angina, obstructive epicardial coronary disease, CMD, or endothelial dysfunction. CMD can be further evaluated by a thorough investigation of the coronary artery response to vasoactive substances including adenosine and acetylcholine to determine if there is endothelial-dependent (decreased coronary blood flow [CBF] with intracoronary acetylcholine) or endothelial-independent dysfunction (decreased CBF with intracoronary adenosine) [71•, 72]. However, a study showed there can be significant overlap between all these mechanisms that can cause angina [73•].
Traditionally, women had higher rates of peri-procedural bleeding and vascular complications with PCI [74••, 75•, 76•], which were strongly associated with higher MACE. The Study of Access Site for Enhancement of Percutaneous Coronary Intervention (SAFE-PCI) demonstrated that radial access site was feasible for women [74••]. Subsequent large RCTs have also supported the radial approach to reduce vascular complications and bleeding [77•].
The Dartmouth Dynamic Registry found that temporal changes in access site techniques and improvements in antithrombotic regimens have led to a decreased risk of bleeding and vascular complications in women. By 2016 there was no longer any difference between the sexes for vascular complications, although women still have higher rates of bleeding with PCI [78•]. Collaboration with interventional colleagues and utilizing this approach is essential in managing women with IHD.
Treatments
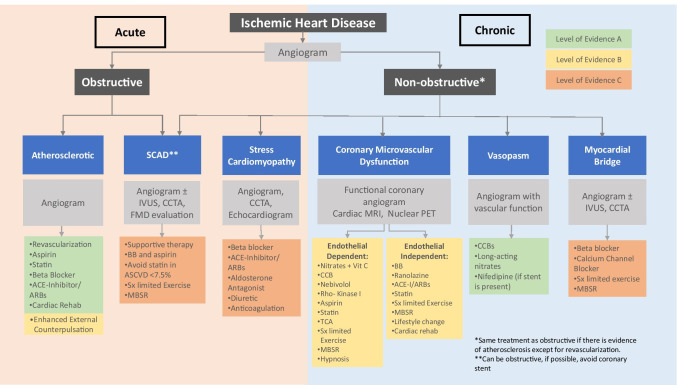
Women are less likely to receive GDMT compared with men with IHD [79•, 80, 81•]. Through research initiatives and the emergence of HCW, there has been an improved understanding of sex-specific diagnosis and treatment of IHD in women. The discontinuation of harmful medications is equally essential for women with IHD. HRT such as estrogen with and without progesterone should be discontinued in the presence of IHD [82]. Research is ongoing to understand the role of HRT, if any, in CVD prevention. GDMT is followed for primary prevention of CVD in women, and these guidelines were summarized in 2020 [83••]. The following summarizes essential treatments for women. The 2014 guideline for stable IHD stated that there is a paucity in GDMT in special populations, such as women [84]. Figure 2 outlines treatment choices based predominantly on expert consensus for these conditions [72, 84, 85••, 86].
Fig. 2.
Evaluation and management of ischemic heart disease with levels of evidence of the treatments. Abbreviations: ACE-I, angiotensin converting enzyme inhibitor; ARBs, angiotensin receptor blockers; BB, beta-blocker; CCB, calcium channel blocker; CCTA, cardiac computed tomography angiography; IVUS, intravascular ultrasound; ENDO PAT, endothelial dysfunction Peripheral Arterial Tone; FMD, fibromuscular dysplasia; MBSR, mindfulness-based stress reduction; MRI, magnetic resonance imaging; PET, positron emission tomography; rehab, rehabilitation; SCAD, spontaneous coronary artery dissection; Sx, symptom; TCA, tricyclic antidepressants
Pharmacologic
Low-dose aspirin (75–100 mg/day) may be considered for primary prevention in select women age 40–70 who are at higher ASCVD risk but who do not have an increased risk of bleeding [17••]. Diabetes alone does not qualify women to be treated with aspirin [80, 87•]. The ACC/AHA 2019 guidelines on treating cholesterol recommended statins depending on their risk of MACE [17••, 88]. Women with a 10-year risk of atherosclerotic CVD (ASCVD) events < 7.5%, should be evaluated for risk-enhancing factors including history of premature menopause (< 40 years); adverse pregnancy outcomes; high-risk race/ethnicity (e.g., South Asian ancestry); persistently elevated, primary hypertriglyceridemia (≥ 175 mg/dL); elevated high-sensitivity C-reactive protein (≥ 2.0 mg/L); and elevated lipoprotein a (Lp(a)) (Lp(a) ≥ 50 mg/dL or ≥ 125 nmol/L). Shared decision-making to start aspirin and statins for primary prevention is recommended by guidelines [40••].
Acute Coronary Syndrome
There are established post-MI medications from RCTs. The 2014 ACC/AHA NSTEMI guidelines give a Class IA indication to use the same pharmacological agents in women and men for acute events and secondary prevention [82]. Delays in GDMT in women have led to an increased rate of readmission, reinfarction, and death in the first year after MI [79•, 80, 81•].
Nonobstructive IHD
Treatment of INOCA includes established anti-ischemic drugs such as nitrates, β-blockers (BB), calcium channel blockers (CCB), and angiotensin-converting enzyme inhibitors (ACEI). Statins are recommended as their anti-inflammatory properties improve endothelial function and angina when combined with anti-ischemic medications [89••]. Ranolazine and aminophylline have shown variable benefits (74). Prinzmetal’s angina is treated with calcium channel blockers and nitrates [90•]. The Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) is currently enrolling 4422 symptomatic women and randomizing them to intensive ACEI, statin, and aspirin therapy vs. usual care to assess all-cause MACE over 3 years [91].
SCAD
Patients presenting with SCAD had better early outcomes with conservative treatment with resolution of the dissection. PCI had higher risk of technical complications and propagation of the intramural hematoma and dissection. CABG was safe and effective in the short term but patients had high rates of graft occlusion due to the dissection resolving. Recurrence of SCAD was seen in 17%, all in women, in one study. These patients are treated with long-term use of aspirin and BB and short-term use of clopidogrel. Beta-blockade has been associated with lowering the risk of recurrence [92••]. Statins are discouraged and recommended only for appropriate ASCVD risk (10-year risk ≥ 7.5%), as they have been associated with recurrent SCAD [93]. There are no RCTs, and these suggestions are mainly based on expert opinion.
Myocardial Bridge
BBs help symptoms by decreasing the artery’s contractility and compression by decreasing heart rate and increasing diastolic filling time. If vasospasm is present, BBs should not be used alone. Nitrates are contraindicated because they worsen symptoms by vasodilating both the proximal and distal portions of the vessel adjacent to the bridge and increase systolic compression of the bridged section [94••]. The first clinical trial with blinded treatment to BB (nebivolol), CCB (diltiazem) vs. placebo for IHD due to myocardial bridging is underway [95•].
Stress Cardiomyopathy
There is a 9:1 female to male preponderance in stress cardiomyopathy, especially in postmenopausal women. Studies have shown that endothelial dysfunction leading to epicardial or microvascular coronary spasm may be the pathogenic mechanism in stress cardiomyopathy [34•]. Anticoagulation is recommended if the left ventricular ejection fraction is < 30% until the akinesis or dyskinesis has resolved or for 3 months, whichever is shorter, and in patients with intraventricular thrombus until resolution [96•].
Stents and CABG
Revascularization, either with PCI or bypass surgery (CABG), improves the quality of life and mortality risk when done in the appropriate clinical scenarios. Women undergoing stenting have more short-term procedural complications than men and suffer higher in-hospital bleeding, death, MI, and vascular complications. Radial vs. femoral access sites significantly decreased these complications in women [74••]. Long-term follow-up after stenting (~ 20 months) shows that women tend to have decreased mortality compared to men [97].
CABG continues to be the treatment of multivessel CAD, especially in those with diabetes. Women undergoing CABG have higher mortality and post-operative complications despite having less coronary plaque burden preoperatively [98]. The increased mortality is more exaggerated at younger ages at the time of CABG than older patients. One large study illustrated a three-fold higher risk of death in women age < 50, despite risk factor adjustment [99]. Alternate surgical techniques, such as off-pump bypass, have been shown to decrease this mortality and respiratory complications in women [100].
Non-pharmacologic Treatments
Cardiac Rehabilitation
Cardiac rehabilitation (CR) has Level 1A class recommendation as a mode of therapy to improve outcomes for patients after a cardiovascular event and cardiac surgery [82]. CR is underused despite substantial evidence-based research; women are in particular under-referred [101]. Of the women who were referred to CR, only 39% of women and 45% of men enroll and adherence was also found to be lower in women than men [102]. As a result, women are under-represented in research regarding CR leading to a paucity in sex-specific outcomes data [103••]. Alternatives to conventional CR, such as virtual/on-demand CR from the convenience of their home, are other options. Home-based CR programs are effective and safe [104•]. Increasing the participation in CR from 20 to 70% would save 25,000 lives and prevent 180,000 hospitalizations annually in the US [105•]. An integrated HCW team approach with established CR referral systems can deliver much-needed care to women with IHD. Additionally, in 2019 a perspective suggested that high-intensity interval training may be of greater benefit for women, with improved cardiovascular outcomes and mental health [103••].
Enhanced External Counterpulsation
Existing data, mainly from uncontrolled studies, suggest a benefit from enhanced external counterpulsation (EECP) has a Class IIb recommendation for patients with angina refractory to other therapy [84]. Additional data from well-designed RCTs are needed to better define this therapeutic strategy’s role in patients with stable IHD.
Multidisciplinary Team
The role of HCW is to leverage multidisciplinary care to personalize IHD treatment for women. Our collective experience in utilizing a vast range of cardiovascular experts in intervention, imaging, HF, and prevention has been successful. Critical to the HCW team also includes primary care, psychology, nutrition, and exercise physiology. The broader team includes administrative, clinical, and research staff to bring translational research to clinical practice. We highlight the roles of a few members below since more detailed components of the programmatic development have been previously published [8••].
Registered dietitian nutritionists (RDNs) provide tailored medical nutrition therapy (MNT) by assessing the patient’s baseline nutrition status, comorbidities, and social determinants of health to provide behavioral change strategies that can lower TC and LDL-C by up to 13% [106••] and reduce relative CVD event risk by 20% [107•]. MNT in dyslipidemia results in cost savings related to reduced physician time, medication use ($638 to $1456 per patient per year), and hospital readmission rates [106••]. RDNs help patients integrate nutrient-based research into whole food-based, clinically and socioeconomically feasible strategies for individual patients. Furthermore, the expansion of telenutrition during the COVID-19 public health emergency has increased patient access to nutrition services integral to heart disease prevention.
Advanced practice providers (APPs) are integral members of the HCW team and often the first to encounter patients to assess and evaluate risk factors for CVD [17••, 40••]. They educate women regarding a heart-healthy diet and exercise, the foundation of cardiovascular health. APPs improve access to care, leading to improved clinical outcomes, more timely interventions, and patient satisfaction [108••].
Psychologists are critical in healthcare delivery at HCW. Women with IHD are more likely than women in the general population to experience psychological difficulties. The accumulation of evidence indicates that behavioral health interventions positively impact cardiac and mental health outcomes in cardiac patients [27••, 29]. Cognitive behavioral therapy is an effective treatment for depression, anxiety, panic disorder, trauma, lifestyle modification, and insomnia [109, 110•, 111]. Mindfulness-based stress reduction (MBSR) and biofeedback have positively affected cardiac conditions such as sinus tachycardia and reactive hypertension [112, 113••]. MBSR can be used in various manifestations of IHD (Fig. 2). Furthermore, behavioral health services embedded in the medical setting are associated with improved identification of behavioral health issues, medical adherence, quality of care, and lower utilization rates of acute healthcare services [114]. HCW address women’s mental health to comprehensively improve CVD prevention, IHD recovery, and patients’ quality of life.
Conclusions
Clinicians of HCW have a critical role in managing IHD in women, which remains the leading cause of CVD mortality. IHD in women is complex, requiring many clinical experts to address individual needs. HCW highlight the multidisciplinary approach utilized to manage these patients though outcomes data for these centers are still being investigated. Table 1 is a summary of this review. Further work to determine the type of IHD and specific treatments is required. Sex-specific risk factors have been identified and multimodality cardiac imaging is improving in diagnosing IHD in women. Treatments are being studied to help improve symptoms and outcomes in women with IHD. There are no evidence-based guidelines in managing the various forms of CAD that are predominantly found in women, resulting from a lack of RCTs. HCW must continue to encourage and empower women to participate in research to determine what diagnostic approach and treatments will work best in improving their quality of life and outcomes.
Table 1.
Summary of the role of heart centers for women in the management of ischemic heart disease
| Role of heart centers for women in the management of ischemic heart disease |
|---|
| Recognition of sex-specific risk factors |
| Adverse pregnancy outcomes |
| Systemic inflammatory disorders |
| Gynecologic cancer therapies |
| Psychosocial factors |
| Lower socioeconomic status |
| Evaluation of ischemic heart disease |
| Use of coronary artery calcium score for intermediate risk women |
| Stress myocardial perfusion imaging for women with abnormal electrocardiograms |
| Photon emission tomography imaging for women to assess coronary flow reserve and coronary microvascular dysfunction |
| Coronary computed tomography angiography to detect and exclude coronary artery disease |
| Cardiovascular magnetic resonance imaging to detect coronary microvascular dysfunction |
| Coronary angiography can be used to diagnose obstructive and non-obstructive coronary artery disease |
| Sex-specific treatments |
| Discontinue harmful medications such as hormone therapy in patients with coronary artery disease |
| Appropriate aspirin and statins for primary prevention |
| Appropriate and optimal use of guideline-directed medical therapy for secondary prevention |
| Identify and mitigate factors that can increase interventional procedural complications |
| Collaborate with cardiac surgeons to consider lower risk surgical techniques such as off pump bypass |
| Emphasize importance of cardiac rehabilitation after cardiovascular events and procedures |
| Multidisciplinary team |
| Emphasize the importance of multidisciplinary team in care of women with coronary artery disease |
Author Contribution
All authors contributed to the literature review, writing, and editing of the document. Dr. Abha Khandelwal consolidated the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Conflict of Interest
No authors have any conflict of interest in writing this paper. The authors have no financial or proprietary interests in any material discussed in this article.
Bakir—consultant Amgen.
Khandelwal—Research Funding Novartis CTQJ.
Volgman—Research funding: NIH IND Number 119127; NIH NINR R01NR018443; Novartis CTQJ230A12001; Stock ownership: Apple, Inc.; MSD/Bayer Virtual Global Advisory Board Member, Bristol Myers Squibb Foundation Diverse Clinical Investigator Career Development Program (DCICDP) National Advisory Committee (NAC).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Women and Ischemic Heart Disease
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abha Khandelwal, Email: akhandel@stanford.edu.
May Bakir, Email: may.bakir@lumc.edu.
Meghan Bezaire, Email: megg_20@hotmail.com.
Briana Costello, Email: Bcostello@texasheart.org.
Joanne Michelle D. Gomez, Email: Joanne.Gomez@cshs.org
Valerie Hoover, Email: vhoover@stanford.edu.
Noreen T. Nazir, Email: nnazir@uic.edu
Katherine Nichols, Email: Knichols6@mgh.Harvard.edu.
Amy Reisenberg, Email: AReisenberg@StanfordHealthcare.org.
Anupama Rao, Email: Anupama_K_Rao@rush.edu.
Rupa Sanghani, Email: Rupa_Sanghani@rush.edu.
Melissa Tracy, Email: Melissa_Tracy@rush.edu.
Annabelle Santos Volgman, Email: Annabelle_Volgman@rush.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.•• Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021: CIR0000000000000950. 10.1161/CIR.0000000000000950. This annual statement paper gives the latest statistical update of mortality and morbidity caused by heart disease and strokes.
- 3.Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med. 1985;313(17):1044–1049. doi: 10.1056/nejm198510243131703. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PWF, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med. 1985;313(17):1038–1043. doi: 10.1056/nejm198510243131702. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses’ Health Study. N Engl J Med. 1991;325(11):756–62. 10.1056/nejm199109123251102. [DOI] [PubMed]
- 6.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280(7):605–13. 10.1001/jama.280.7.605. [DOI] [PubMed]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg GP, Mehta LS, Sanghani RM, Patel HN, Aggarwal NR, Aggarwal NT, et al. Heart centers for women: historical perspective on formation and future strategies to reduce cardiovascular disease. Circulation. 2018;138(11):1155–1165. doi: 10.1161/circulationaha.118.035351. [DOI] [PubMed] [Google Scholar]
- 9.National Heart Lung and Blood Institute. Women’s heart health: developing a national health education action plan (No. 01–2963). Office of Prevention, Education, and Control: Silver Spring, MD, USA. 2001.
- 10.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, et al. Evidence-based guidelines for cardiovascular disease prevention in women. J Am Coll Cardiol. 2004;43(5):900–921. doi: 10.1016/j.jacc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Peters SAE, Colantonio LD, Dai Y, Zhao H, Bittner V, Farkouh ME, Dluzniewski P, Poudel B, Muntner P, Woodward M. Trends in recurrent coronary heart disease after myocardial infarction among US women and men between 2008 and 2017. Circulation. 2021;143(7):650–660. doi: 10.1161/CIRCULATIONAHA.120.047065. [DOI] [PubMed] [Google Scholar]
- 12.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathak LA, Shirodkar S, Ruparelia R, Rajebahadur J. Coronary artery disease in women. Indian Heart J. 2017;69(4):532–538. doi: 10.1016/j.ihj.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehilli J, Presbitero P. Coronary artery disease and acute coronary syndrome in women. Heart. 2020;106(7):487–492. doi: 10.1136/heartjnl-2019-315555. [DOI] [PubMed] [Google Scholar]
- 15.Maas AH, Appelman YE. Gender differences in coronary heart disease. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2010;18(12):598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol. 2014;114(2):187–192. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi: 10.1161/cir.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geraghty L, Figtree GA, Schutte AE, Patel S, Woodward M, Arnott C. Cardiovascular disease in women: from pathophysiology to novel and emerging risk factors. Heart Lung Circ. 2021;30(1):9–17. doi: 10.1016/j.hlc.2020.05.108. [DOI] [PubMed] [Google Scholar]
- 19.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamad R, Penko J, Kazi DS, Coxson P, Guzman D, Wei PC, et al. Association of low socioeconomic status with premature coronary heart disease in US adults. JAMA cardiology. 2020 doi: 10.1001/jamacardio.2020.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw LJ, Merz CN, Bittner V, Kip K, Johnson BD, Reis SE et al. Importance of socioeconomic status as a predictor of cardiovascular outcome and costs of care in women with suspected myocardial ischemia. Results from the National Institutes of Health, National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Journal of women’s health. 2008;17(7):1081–92. 10.1089/jwh.2007.0596. [DOI] [PMC free article] [PubMed]
- 22.Creamer J, Mohanty A. United States Census Bureau. U.S. Poverty rate drops to 11.8% in 2018. https://www.census.gov/library/stories/2019/09/poverty-rate-for-people-in-female-householder-families-lowest-on-record.html. September 10, 2019. Accessed February 16 2021.
- 23.Safford MM, Reshetnyak E, Sterling MR, Richman JS, Muntner PM, Durant RW, et al. Number of social determinants of health and fatal and nonfatal incident coronary heart disease in the REGARDS Study. Circulation. 2021;143(3):244–253. doi: 10.1161/CIRCULATIONAHA.120.048026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palacio A, Mansi R, Seo D, Suarez M, Garay S, Medina H, et al. Social determinants of health score: does it help identify those at higher cardiovascular risk? Am J Manag Care. 2020;26(10):e312–e318. doi: 10.37765/ajmc.2020.88504. [DOI] [PubMed] [Google Scholar]
- 25.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(9):873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 26.White-Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e841–e863. doi: 10.1161/CIR.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 27.•• Levine GN, Cohen BE, Commodore-Mensah Y, Fleury J, Huffman JC, Khalid U et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021:CIR0000000000000947. 10.1161/CIR.0000000000000947. This scientific statement presents evidence that psychological health contributes to and causes CVD and that improving psychological health leads to better cardiovascular health. The paper offers simple screening measures that can be utilized by clinicians to assess psychological health and what tools can be used to help the patient. [DOI] [PubMed]
- 28.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanmugasegaram S, Russell KL, Kovacs AH, Stewart DE, Grace SL. Gender and sex differences in prevalence of major depression in coronary artery disease patients: a meta-analysis. Maturitas. 2012;73(4):305–311. doi: 10.1016/j.maturitas.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orth-Gomer K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: the Stockholm Women’s Intervention Trial for Coronary Heart Disease (SWITCHD) Circ Cardiovasc Qual Outcomes. 2009;2(1):25–32. doi: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 31.Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svardsudd K. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: Secondary Prevention in Uppsala Primary Health Care project (SUPRIM) Arch Intern Med. 2011;171(2):134–140. doi: 10.1001/archinternmed.2010.510. [DOI] [PubMed] [Google Scholar]
- 32.Naderi S, Tsai SA, Khandelwal A. Hypertensive disorders of pregnancy. Curr Atheroscler Rep. 2017;19(3):15. doi: 10.1007/s11883-017-0648-z. [DOI] [PubMed] [Google Scholar]
- 33.•• American College of Obstetricians and Gynecologists’ Presidential Task Force on P, Heart D, Committee on Practice B-O. ACOG Practice Bulletin No. 212: pregnancy and heart disease. Obstetrics and gynecology. 2019;133(5):e320-e56. 10.1097/AOG.0000000000003243. This report is from a great summary of the prevalence of heart disease during pregnancy and offers clinicians a comprehensive interpregnancy care plan for pregnant women with heart disease.
- 34.Patel H, Aggarwal NT, Rao A, Bryant E, Sanghani RM, Byrnes M, et al. Microvascular disease and small-vessel disease: the nexus of multiple diseases of women. Journal of women’s health. 2020;29(6):770–779. doi: 10.1089/jwh.2019.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med. 2015;25(2):140–151. doi: 10.1016/j.tcm.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim ESH. Spontaneous coronary-artery dissection. N Engl J Med. 2020;383(24):2358–2370. doi: 10.1056/NEJMra2001524. [DOI] [PubMed] [Google Scholar]
- 37.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303(16):1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, et al. Sex differences in demographics, risk factors, presentation, and noninvasive testing in stable outpatients with suspected coronary artery disease: insights from the PROMISE trial. JACC Cardiovasc Imaging. 2016;9(4):337–346. doi: 10.1016/j.jcmg.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39(41):3727–3735. doi: 10.1093/eurheartj/ehy534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Messenger B, Li D, Nasir K, Carr JJ, Blankstein R, Budoff MJ. Coronary calcium scans and radiation exposure in the multi-ethnic study of atherosclerosis. Int J Cardiovasc Imaging. 2016;32(3):525–529. doi: 10.1007/s10554-015-0799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.deGoma EM, Karlsberg RP, Judelson DR, Budoff MJ. The underappreciated impact of heart disease. Women’s health issues: official publication of the Jacobs Institute of Women’s Health. 2010;20(5):299–303. doi: 10.1016/j.whi.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Dolor RJ, Patel MR, Melloni C, Chatterjee R, McBroom AJ, Musty MD et al. Noninvasive technologies for the diagnosis of coronary artery disease in women. AHRQ Comparative Effectiveness Reviews. Rockville (MD)2012. [PubMed]
- 44.Aggarwal NR, Patel HN, Mehta LS, Sanghani RM, Lundberg GP, Lewis SJ, et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ Cardiovasc Qual Outcomes. 2018;11(2):e004437. doi: 10.1161/CIRCOUTCOMES.117.004437. [DOI] [PubMed] [Google Scholar]
- 45.Standbridge K, Reyes E. The role of pharmacological stress testing in women. Journal of nuclear cardiology: official publication of the American Society of Nuclear Cardiology. 2016;23(5):997–1007. doi: 10.1007/s12350-016-0602-4. [DOI] [PubMed] [Google Scholar]
- 46.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63(4):380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Mieres JH, Shaw LJ, Arai A, Budoff MJ, Flamm SD, Hundley WG, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention. American Heart Association Circulation. 2005;111(5):682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 48.Taqueti VR, Dorbala S, Wolinsky D, Abbott B, Heller GV, Bateman TM, et al. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease-state-of-the-evidence and clinical recommendations. Journal of nuclear cardiology: official publication of the American Society of Nuclear Cardiology. 2017;24(4):1402–1426. doi: 10.1007/s12350-017-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation. 2014;130(4):350–379. doi: 10.1161/CIR.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 50.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 53.Truong QA, Rinehart S, Abbara S, Achenbach S, Berman DS, Bullock-Palmer R, et al. Coronary computed tomographic imaging in women: an expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2018;12(6):451–466. doi: 10.1016/j.jcct.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383–91. 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed]
- 56.Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps) J Am Coll Cardiol. 2014;63(12):1145–1155. doi: 10.1016/j.jacc.2013.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Foy AJ, Dhruva SS, Peterson B, Mandrola JM, Morgan DJ, Redberg RF. Coronary computed tomography angiography vs functional stress testing for patients with suspected coronary artery disease: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(11):1623–1631. doi: 10.1001/jamainternmed.2017.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stocker TJ, Deseive S, Leipsic J, Hadamitzky M, Chen MY, Rubinshtein R, et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI) Eur Heart J. 2018;39(41):3715–3723. doi: 10.1093/eurheartj/ehy546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning T, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J. 2016;37(15):1232–1243. doi: 10.1093/eurheartj/ehv700. [DOI] [PubMed] [Google Scholar]
- 60.Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(24):2993–2999. doi: 10.1161/01.Cir.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 61.•• Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and no obstructive coronary artery disease (INOCA): what is the risk? Journal of the American Heart Association. 2018;7(17):e008868. 10.1161/jaha.118.008868. This paper highlights the lack and need for evidence-based treatment guidelines for patients with INOCA. [DOI] [PMC free article] [PubMed]
- 62.Doyle M, Weinberg N, Pohost GM, Bairey Merz CN, Shaw LJ, Sopko G, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging. 2010;3(10):1030–1036. doi: 10.1016/j.jcmg.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pathik B, Raman B, Mohd Amin NH, Mahadavan D, Rajendran S, McGavigan AD, et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2016;17(10):1146–1152. doi: 10.1093/ehjci/jev289. [DOI] [PubMed] [Google Scholar]
- 64.Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, Mahmud E, Wei J, Marzo K, Matsumura M, Seno A, Hausvater A, Giesler C, Jhalani N, Toma C, Har B, Thomas D, Mehta LS, Trost J, Mehta PK, Ahmed B, Bainey KR, Xia Y, Shah B, Attubato M, Bangalore S, Razzouk L, Ali ZA, Merz NB, Park K, Hada E, Zhong H, Hochman JS. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143(7):624–640. doi: 10.1161/CIRCULATIONAHA.120.052008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131(12):1054–1060. doi: 10.1161/circulationaha.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, et al. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166(1):134–141. doi: 10.1016/j.ahj.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med. 1967;276(19):1063–1066. doi: 10.1056/nejm196705112761904. [DOI] [PubMed] [Google Scholar]
- 69.Reynolds HR, Srichai MB, Iqbal SN, Slater JN, Mancini GB, Feit F, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation. 2011;124(13):1414–1425. doi: 10.1161/circulationaha.111.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23(6):511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, et al. Assessment of vascular dysfunction in patients without obstructive coronary artery disease: why, how, and when. JACC Cardiovasc Interv. 2020;13(16):1847–1864. doi: 10.1016/j.jcin.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med. 2010;12(4):355–364. doi: 10.1007/s11936-010-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar S, Mehta PK, Eshtehardi P, Hung OY, Koh JS, Kumar A, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2020 doi: 10.1002/ccd.29237. [DOI] [PubMed] [Google Scholar]
- 74.Rymer JA, Kaltenbach LA, Kochar A, Hess CN, Gilchrist IC, Messenger JC, et al. Comparison of rates of bleeding and vascular complications before, during, and after trial enrollment in the SAFE-PCI trial for women. Circ Cardiovasc Interv. 2019;12(5):e007086. doi: 10.1161/circinterventions.118.007086. [DOI] [PubMed] [Google Scholar]
- 75.Alkhouli M, Alqahtani F, Elsisy MF, Kawsara A, Alasnag M. Incidence and outcomes of acute ischemic stroke following percutaneous coronary interventions in men versus women. Am J Cardiol. 2020;125(3):336–340. doi: 10.1016/j.amjcard.2019.10.045. [DOI] [PubMed] [Google Scholar]
- 76.Kosmidou I, Leon MB, Zhang Y, Serruys PW, von Birgelen C, Smits PC, et al. Long-term outcomes in women and men following percutaneous coronary intervention. J Am Coll Cardiol. 2020;75(14):1631–1640. doi: 10.1016/j.jacc.2020.01.056. [DOI] [PubMed] [Google Scholar]
- 77.Guo Y, Yin F, Fan C, Wang Z. Gender difference in clinical outcomes of the patients with coronary artery disease after percutaneous coronary intervention: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97(30):e11644. doi: 10.1097/md.0000000000011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaudry HI, Lee J, Li SX, Gasperetti A, Lee KM, Zbib NH et al. Sex differences in acute bleeding and vascular complications following percutaneous coronary intervention between 2003 and 2016: trends from the Dartmouth Dynamic Registry. Cardiovascular revascularization medicine : including molecular interventions. 2020.10.1016/j.carrev.2020.07.028. This registry of consecutive percutaneous coronary interventions (PCI) found that the incidence of bleeding and vascular complications fell between 2003 and 2016 in both men and women and there was no longer any difference between the sexes for this outcome. Although bleeding following PCI has decreased in both sexes over time, women compared to men, continued to have more bleeding. [DOI] [PubMed]
- 79.Zhao M, Vaartjes I, Graham I, Grobbee D, Spiering W, Klipstein-Grobusch K, et al. Sex differences in risk factor management of coronary heart disease across three regions. Heart. 2017;103(20):1587–1594. doi: 10.1136/heartjnl-2017-311429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118(8):1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hyun KK, Redfern J, Patel A, Peiris D, Brieger D, Sullivan D, et al. Gender inequalities in cardiovascular risk factor assessment and management in primary healthcare. Heart. 2017;103(7):492–498. doi: 10.1136/heartjnl-2016-310216. [DOI] [PubMed] [Google Scholar]
- 82.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(20):2602–2618. doi: 10.1016/j.jacc.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(18):1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 85.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 86.Thomas GR, DiFabio JM, Gori T, Parker JD. Once daily therapy with isosorbide-5-mononitrate causes endothelial dysfunction in humans: evidence of a free-radical-mediated mechanism. J Am Coll Cardiol. 2007;49(12):1289–1295. doi: 10.1016/j.jacc.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 87.Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036–1046. doi: 10.1016/S0140-6736(18)31924-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25 Pt B):2889–934. 10.1016/j.jacc.2013.11.002. [DOI] [PubMed]
- 89.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116(4):856–870. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hubert A, Seitz A, Pereyra VM, Bekeredjian R, Sechtem U, Ong P. Coronary artery spasm: the interplay between endothelial dysfunction and vascular smooth muscle cell hyperreactivity. European cardiology. 2020;15:e12. doi: 10.15420/ecr.2019.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). January 31, 2018: ClinicalTrials.gov Identifier: NCT03417388 Women’s IschemiA TRial to Reduce Events In Non-ObstRuctive CAD (WARRIOR). Available from: https://clinicaltrials.gov/ct2/show/NCT03417388.2018.
- 92.Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70(9):1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 93.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 94.Teragawa H, Oshita C, Ueda T. The myocardial bridge: potential influences on the coronary artery vasculature. Clinical Medicine Insights Cardiology. 2019;13:1179546819846493. doi: 10.1177/1179546819846493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.• ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). October 17, 2019. Identifier: NCT04130438 efficacy of medical therapy in women and men with angina and myocardial bridging. Available from: https://clinicaltrials.gov/ct2/show/NCT04130438. 2019. Accessed February 16 2021. This is a clinical trial enrolling patients with myocardial bridging and randomizing them to nebivolol, diltiazem or placebo.
- 96.• Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. Journal of the American College of Cardiology. 2018;72(16):1955–71. 10.1016/j.jacc.2018.07.072. This is a review of the current presentation, diagnosis, and management of patients with stress cardiomyopathy. [DOI] [PMC free article] [PubMed]
- 97.Anderson ML, Peterson ED, Brennan JM, Rao SV, Dai D, Anstrom KJ, et al. Short- and long-term outcomes of coronary stenting in women versus men: results from the National Cardiovascular Data Registry Centers for Medicare & Medicaid services cohort. Circulation. 2012;126(18):2190–2199. doi: 10.1161/circulationaha.112.111369. [DOI] [PubMed] [Google Scholar]
- 98.Edwards FH, Carey JS, Grover FL, Bero JW, Hartz RS. Impact of gender on coronary bypass operative mortality. Ann Thorac Surg. 1998;66(1):125–131. doi: 10.1016/s0003-4975(98)00358-0. [DOI] [PubMed] [Google Scholar]
- 99.Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105(10):1176–1181. doi: 10.1161/hc1002.105133. [DOI] [PubMed] [Google Scholar]
- 100.Brown PP, Mack MJ, Simon AW, Battaglia S, Tarkington L, Horner S et al. Outcomes experience with off-pump coronary artery bypass surgery in women. The Annals of thoracic surgery. 2002;74(6):2113–9; discussion 20. 10.1016/s0003-4975(02)03988-7. [DOI] [PubMed]
- 101.Samayoa L, Grace SL, Gravely S, Scott LB, Marzolini S, Colella TJ. Sex differences in cardiac rehabilitation enrollment: a meta-analysis. Can J Cardiol. 2014;30(7):793–800. doi: 10.1016/j.cjca.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Bennett AL, Lavie CJ, Grace SL. Cardiac rehabilitation following acute coronary syndrome in women. Curr Treat Options Cardiovasc Med. 2017;19(8):57. doi: 10.1007/s11936-017-0559-x. [DOI] [PubMed] [Google Scholar]
- 103.Way KL, Reed JL. Meeting the needs of women in cardiac rehabilitation. Circulation. 2019;139(10):1247–1248. doi: 10.1161/circulationaha.118.037754. [DOI] [PubMed] [Google Scholar]
- 104.• Choxi R, Kolominsky J, Al Rifai M, Patel J, Shapiro MD. Cardiac rehabilitation and implications during the COVID-19 era https://www.acc.org/latest-in-cardiology/articles/2021/01/04/14/03/cardiac-rehabilitation-and-implications-during-the-covid-19-era. Accessed 1/24/21. This is an expert analysis on CR during COVID times looking at home-based cardiac rehab vs. facility based.
- 105.Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92(2):234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sikand G, Cole RE, Handu D, deWaal D, Christaldi J, Johnson EQ, et al. Clinical and cost benefits of medical nutrition therapy by registered dietitian nutritionists for management of dyslipidemia: a systematic review and meta-analysis. J Clin Lipidol. 2018;12(5):1113–1122. doi: 10.1016/j.jacl.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 107.O’Connor EA, Evans CV, Rushkin MC, Redmond N, Lin JS. Behavioral counseling to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;324(20):2076–2094. doi: 10.1001/jama.2020.17108. [DOI] [PubMed] [Google Scholar]
- 108.Woo BFY, Lee JXY, Tam WWS. The impact of the advanced practice nursing role on quality of care, clinical outcomes, patient satisfaction, and cost in the emergency and critical care settings: a systematic review. Hum Resour Health. 2017;15(1):63. doi: 10.1186/s12960-017-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olatunji BO, Kauffman BY, Meltzer S, Davis ML, Smits JA, Powers MB. Cognitive-behavioral therapy for hypochondriasis/health anxiety: a meta-analysis of treatment outcome and moderators. Behav Res Ther. 2014;58:65–74. doi: 10.1016/j.brat.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Reavell J, Hopkinson M, Clarkesmith D, Lane DA. Effectiveness of cognitive behavioral therapy for depression and anxiety in patients with cardiovascular disease: a systematic review and meta-analysis. Psychosom Med. 2018;80(8):742–753. doi: 10.1097/PSY.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 111.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 112.Mikosch P, Hadrawa T, Laubreiter K, Brandl J, Pilz J, Stettner H, et al. Effectiveness of respiratory-sinus-arrhythmia biofeedback on state-anxiety in patients undergoing coronary angiography. J Adv Nurs. 2010;66:1101–1110. doi: 10.1111/j.1365-2648.2010.05277.x. [DOI] [PubMed] [Google Scholar]
- 113.Lee EKP, Yeung NCY, Xu Z, Zhang D, Yu C-P, Wong SYS. Effect and acceptability of mindfulness-based stress reduction program on patients with elevated blood pressure or hypertension. Hypertension. 2020;76(6):1992–2001. doi: 10.1161/HYPERTENSIONAHA.120.16160. [DOI] [PubMed] [Google Scholar]
- 114.Reiss-Brennan B, Brunisholz KD, Dredge C, Briot P, Grazier K, Wilcox A, et al. Association of integrated team-based care with health care quality, utilization, and cost. JAMA. 2016;316(8):826–834. doi: 10.1001/jama.2016.11232. [DOI] [PubMed] [Google Scholar]