Abstract
The increase of eosinophil levels is a hallmark of type-2 inflammation. Blood eosinophil counts act as a convenient biomarker for asthma phenotyping and the selection of biologics, and they are even used as a prognostic factor for severe coronavirus disease 2019. However, the circulating eosinophil count does not always reflect tissue eosinophilia and vice versa. The mismatch of blood and tissue eosinophilia can be seen in various clinical settings. For example, blood eosinophil levels in patients with acute eosinophilic pneumonia are often within normal range despite the marked symptoms and increased number of eosinophils in bronchoalveolar lavage fluid. Histological studies using immunostaining for eosinophil granule proteins have revealed the extracellular deposition of granule proteins coincident with pathological conditions, even in the absence of a significant eosinophil infiltrate. The marked deposition of eosinophil granule proteins in tissue is often associated with cytolytic degranulation. Recent studies have indicated that extracellular trap cell death (ETosis) is a major mechanism of cytolysis. Cytolytic ETosis is a total cell degranulation in which cytoplasmic and nuclear contents, including DNA and histones that act as alarmins, are also released. In the present review, eosinophil-mediated inflammation in such mismatch conditions is discussed.
Keywords: Eosinophil granule proteins, Eosinophils, Extracellular traps
INTRODUCTION
Allergy, a hypersensitivity reaction initiated by specific immunologic mechanisms [1], is often associated with tissue eosinophilia [2]. In addition, eosinophil accumulation is also associated with malignancy, infection, and various homeostatic conditions. For example, eosinophils home into the uterus, which is regulated by the estrus cycle [3,4]. Eosinophils in the blood can be readily counted. Although these cells form a minor (<5%) component of the circulating leukocyte population in the blood, larger numbers of tissue-dwelling eosinophils are present outside of the vasculature [5]. Nevertheless, the blood eosinophil count is an easy-to-access biomarker for various clinical applications. For asthma phenotyping and the selection of biologics, the blood eosinophil count is the best-established biomarker [6]. In patients with severe chronic obstructive diseases, blood eosinophil counts are associated with the risk of exacerbations and the benefit of inhaled corticosteroid use [7]. A lower blood eosinophil count is also useful as a poor prognostic factor for severe coronavirus disease 2019 (COVID-19) [8,9].
Eosinophils develop in the bone marrow and are released into blood circulation once they are maturated. Circulating eosinophils can subsequently transmigrate into the gastrointestinal tract, lungs, adipose tissue, thymus, spleen, lymph nodes, and mammary glands, where they exert various essential homeostatic functions [10,11]. The life span of eosinophils is unclear, but it has been estimated to be less than 1 week under homeostatic conditions [12]. The tissue presence of eosinophils is determined by their recruitment, retention, and clearance [13]. Therefore, tissue eosinophilia can be caused by increased migration, prolonged survival, impaired phagocytic clearance, or decreased luminal entry [14].
The activation status of eosinophils is mainly tuned by receptors expressed on their cell surfaces. As a short-lived, nondividing cell, eosinophil “activation” has been recognized as the release of bioactive mediators into the extracellular milieu [15]. This is in contrast to lymphocytes, for which “activation” usually means proliferation and clonal expansion of antigen-specific lymphocytes [16]. The multifaceted role of eosinophils is evidenced by their range of cell densities and cell-derived mediators.
SECRETORY MECHANISMS OF EOSINOPHILS
Because eosinophils are a rich source of bioactive mediators, the simplified hypothesis is that the amount of eosinophil-derived inflammatory mediators in the microenvironment is the primary cause of eosinophilic inflammation. Eosinophils contain approximately 200 granules per cell [17]. These granules contain 4 major cationic (basic) proteins, that is, major basic protein (MBP), eosinophil-derived neurotoxin (EDN), eosinophil cationic protein (ECP), and eosinophil peroxidase (EPO), which play important roles in eosinophil-mediated inflammation [18]. Granule-stored cytokines in human eosinophils are released by 3 secretory processes: classical exocytosis, piecemeal degranulation, and cytolysis/extracellular trap cell death (ETosis) [5,19].
Classical exocytosis is a granule secretory system in which intracellular granules fuse with the plasma membrane and release their entire contents extracellularly via a secretory pore. This mechanism is not usually observed in vivo. In compound exocytosis, granules fuse to each other, forming large channels within the cytoplasm, and the cells then secrete the entire intragranular contents of multiple granules [18].
Piecemeal degranulation is thought to be the main degranulation process used by eosinophils. In piecemeal degranulation, the granule contents are selectively mobilized into small round vesicles and tubular structures termed eosinophil sombrero vesicles. The tubular eosinophil sombrero vesicles express cytokine receptor chains that are bound by cytokine ligands, such as interleukin (IL)-4 [5]. These vesicles become fused with the plasma membrane and release their granule-derived contents into extracellular spaces [20,21]. Ultrastructure of exocytosis and piecemeal degranulation show an emptying of the secretory granules or lucent areas in the core of intact eosinophils [18].
Cytolysis or ETosis is a recently recognized type of programmed cell death that is characterized by the dissolution of nuclear and plasma membranes and release of chromatin fiber called an extracellular trap [22,23,24]. In eosinophils, ETosis-derived cell-free extracellular granules express functional receptors on their membranes [18,19,25,26]. Upon activation by ligand stimulation, the granules release their contents, which include EDN [27,28]. Eosinophil ETosis (EETosis) is considered to contribute to the sterilization and trapping of pathogens by extracellular traps, granule proteins, and nuclear-derived components, all of which have cytotoxicity [29,30].
BIOACTIVE MEDIATORS OF EOSINOPHILS
The functions of the major mediators derived from human eosinophils are summarized in Table 1. MBP is localized in the core of specific granules, while EDN, ECP, and EPO are localized in the matrix of specific granules [31,32,33]. Although all 4 of these granule proteins have toxicity for helminth parasites [34], each has various biological activities in addition to its antimicrobial activity. MBP-2 and EPO are distributed only in eosinophils [35,36], whereas the other granule proteins are found in additional cells and tissues [37,38,39]. Nevertheless, these granule proteins characterize eosinophil-mediated inflammation with their abundance.
Table 1. Eosinophil-derived mediators.
| Eosinophil | Mediators | Cell distribution | Functions | Selected references |
|---|---|---|---|---|
| Eosinophil-specific | MBP1/2 | Granule core | Toxic to cells and tissues | Gleich GJ, et al. J Immunol 1979;123:2925–7. [46] |
| Toxic to helminth parasites | Hamann KJ, et al. J Immunol 1990;144:3166–73. [34] | |||
| Butterworth AE, et al. J Immunol 1979;122:221–9. [162] | ||||
| Toxic to bacteria | Lehrer RI, et al. J Immunol 1989;142:4428–34. [41] | |||
| Disrupts the lipid bilayer membrane | Gleich GJ, et al. Annu Rev Med 1993;44:85–101. [163] | |||
| Inhibits the Muscaline 2 receptor | Jacoby DB, et al. J Clin Invest 1993;91:1314–8. [49] | |||
| Induces mediator release from basophils and mast cells | O'Donnell MC, et al. J Exp Med 1983;157:1981–91. [42] | |||
| Fujisawa D, et al. J Allergy Clin Immunol 2014;134:622–33.e9. [43] | ||||
| Zheutlin LM, et al. Int Arch Allergy Appl Immunol 1985;77:216–7. [164] | ||||
| Induces IL-8 production from eosinophils | Kita H, et al. J Immunol 1995;154:4749–58. [45] | |||
| Induces detachment of tracheal epithelial cells | Hastie AT, et al. Am Rev Respir Dis 1987;135:848–53. [47] | |||
| Induces cessation of ciliary activity | ||||
| Induces platelet activation | Rohrbach MS, et al. J Exp Med 1990;172:1271–4. [44] | |||
| Induces amyloid deposition | Soragni A, et al. Mol Cell 2015;57:1011–21. [165] | |||
| EDN | Granule matrix | Toxic to helminth parasites | Hamann KJ, et al. J Immunol 1990;144:3166–73. [34] | |
| Has RNase2 activity | Slifman NR, et al. J Immunol 1986;137:2913–7. [166] | |||
| Induces the Gordon phenomenon | Fredens K, et al. J Allergy Clin Immunol 1982;70:361–6. [167] | |||
| Induces dendritic cell activation | Yang D, et al. J Exp Med 2008;205:79–90. [57] | |||
| Induces MMP9 expression and apoptosis in keratinocytes | Amber KT, et al. Exp Dermatol 2018;27:1322–7. [67] | |||
| ECP | Granule matrix | Has RNase3 activity | Gullberg U, et al. Biochem Biophys Res Commun 1986;139:1239–42. [61] | |
| Slifman NR, et al. J Immunol 1986;137:2913–7. [166] | ||||
| Induces the Gordon phenomenon | Fredens K, et al. J Allergy Clin Immunol 1982;70:361–6. [167] | |||
| Toxic to helminth parasites | McLaren DJ, et al. Parasite Immunol 1981;3:359–73. [62] | |||
| Hamann KJ, et al. J Parasitol 1987;73:523–9. [54] | ||||
| Cytotoxic | Rosenberg HF and Dyer KD. J Biol Chem 1995;270:7876–81. [168] | |||
| Toxic to bacteria | Lehrer RI, et al. J Immunol 1989;142:4428–34. [41] | |||
| Induces amyloid-like aggregation | Torrent M, et al. PLoS Pathogens 2012;8:e1003005. [56] | |||
| Induces mediator release from basophils and mast cells | Zheutlin LM, et al. Int Arch Allergy Appl Immunol 1985;77:216–7. [164] | |||
| Inhibits TNF-α production by human macrophages | Pulido D, et al. FEBS J 2016;283:4176–91. [60] | |||
| Induces MMP9 expression and apoptosis in keratinocytes | Amber KT, et al. Exp Dermatol 2018;27:1322–7. [67] | |||
| Induces plasminogen activation | ||||
| Enhances factor XII-dependent reactions | Dahl R and Venge P. Thromb Res 1979;14:599–608. [65] | |||
| Venge P, et al. Thromb Res 1979;14:641–9. [64] | ||||
| EPO | Granule matrix | Toxic to helminth parasites | Hamann KJ, et al. J Immunol 1990;144:3166–73. [34] | |
| Toxic to bacteria | Migler R, et al. Blood 1978;51:445–56. [75] | |||
| Wang JG, et al. Blood 2006;107:558–65. [77] | ||||
| Toxic to tumor cells | Jong EC and Klebanoff SJ. J Immunol 1980;124:1949–53. [76] | |||
| Induces tissue factor and thrombosis | Wang JG, et al. Blood 2006;107:558–65. [77] | |||
| Binds to the surface of microorganisms and enbhances phagocytosis | Ramsey PG, et al. J Immunol 1982;128:415–20. [78] | |||
| Mediates mucus plugging of the airways | Dunican EM, et al. J Clin Invest 2018;128:3:997–1009 [79] | |||
| Inactivates of leukotrienes | Henderson WR, et al. J Immunol 1982;128:2609–13. [81] | |||
| Induces histamine release from mast cells | Henderson WR, et al. J Exp Med 1980;152:265–79. [80] | |||
| Fujisawa D, et al. J Allergy Clin Immunol 2014;134:622–33.e9 [43]. | ||||
| Galectin-10 | Peripheral cytoplasm | Forms Charcot-Leyden crystals | Ueki S, et al. Blood 2018;132:2183–7. [84] | |
| Promotes allergic inflammation | Persson EK, et al. Science 2019;364:4295. [85] | |||
| Regulates the proliferative capacity and suppressive function of CD25+ Treg cells | Kubach J, et al. Blood 2007;1;110:1550–8. [169] | |||
| Involved in the secretory response via lysophospholipase activity | Weller PF, et al. J Leukoc Biol. 2020;108:105–12. [87] | |||
| Induces granuleogenesis and vesicular transport of granule proteins | Grozdanovic MM, et al. J Allergy Clin Immunol 2020;146:377–89.e10. [88] | |||
| Nonspecific for eosinophil | Histones | Nucleus | Promotes apoptosis | Barrero CA, et al. Am J Respir Crit Care Med 2013;188:673–83. [91] |
| Gilthorpe JD, et al. F1000Res 2013;2:148. [92] | ||||
| Stimulates neurogenesis | Mishra B, et al. J Neurosci 2010;30:12400–13. [170] | |||
| Regulates macrophage migration and endocytosis | Brix K, et al. J Clin Invest 1998;102:283–93. [171] | |||
| Regulates neutrophil migration | Xu J, et al. Nat Med 2009;15:1318–21. (in mice) [172] | |||
| Cytotoxic to endothelial cells | Bosmann M, et al. Faseb J 2013;27:5010–21. [173] | |||
| Allam R, et al. J Am Soc Nephrol 2012;23:1375–88. [174] | ||||
| Toxic to bacteria | Hirsch JG. J Exp Med 1958;108:925–44. [90] | |||
| Stimulates TLR signaling pathways | Huang H, et al. Hepatology 2011;2:54:999–1008. [93] | |||
| Semeraro F, et al. Blood 2011;118:1952–61. [95] | ||||
| Kawano H, et al. Lab Invest 2014;94:569–85. [94] | ||||
| Induces NLRP3 Inflammasome activation | Allam R, et al. Eur J Immunol 2013;43:3336–42. [96] | |||
| Huang H, et al. J Immunol 2013;191:2665–79. [97] | ||||
| Promotes thorombin generation | Fuchs TA, et al. Blood 2011;118:3708–14. (in mice) [98] | |||
| Ammollo CT, et al. J Thromb Haemost 2011;9:1795–1803. [99] | ||||
| Induces platelet aggregation | Lam FW, et al. Thromb Res 2013;132:69–76. [101] | |||
| Carestia A,et al. Thromb Haemost 2013;110:1035–45. [100] | ||||
| dsDNA | Nucleus | Contributes to microbial pathogen containment | Kaplan MJ and Radic M. J Immunol 2012;189:2689–95. [89] | |
| Stimulates autoimmune responses | Garcia-Romo GS, et al. Sci Transl Med 2011;3:73ra20. [104] | |||
| Kessenbrock K, et al. Nat Med 2009;15:623–25. [105] | ||||
| Hakkim A, et al. Proc Natl Acad Sci USA 2010;107:9813–8. [175] | ||||
| Lande R, et al. Sci Transl 2011;Med3:73ra1. [106] | ||||
| Induces vascular damage | Villanueva E, et al. J Immunol 2011;187: 538–52. [102] | |||
| Gupta AK, et al. FEBS Lett 2010;584:3193–3. [103] | ||||
| Promotes thorombosis | Fuchs TA, et al. Proc Natl Acad Sci USA 2010;107:15880–5. [176] |
MBP1/2, major basic protein 1/2; IL-8, interleukin-8; EDN, eosinophil-derived neurotoxin; MMP9, matrix metallopeptidase 9; ECP, eosinophil cationic protein; TNF, tumor necrosis factor; EPO, eosinophil peroxidase; TLR, Toll-like receptor; dsDNA, double-stranded DNA
Major basic protein
There are 2 types of MBP: MBP-1 and MBP-2. MBP-1 is more potent and more widespread compared with its homologue MBP-2, which is only present in eosinophils [35]. MBP is rich in arginine and has strong basicity [40]. It mediates cytotoxicity for bacteria by increasing the permeability of cell membranes [41]. Both MBP and EPO induce histamine release from basophils and mast cells, and histamine release in mast cells occurs through the Mas-related gene X2 receptor [42,43]. MBP is also known to be involved in the activation of neutrophils and platelets [44] and to induce IL-8 production by eosinophils themselves [45]. According to an in vitro study, MBP causes damage to various cells and tissues, such as the intestine, spleen, skin, and tracheal epithelium [46]. Furthermore, MBP induces the detachment of airway epithelium and arrest of ciliary activity, which may be related to the pathogenesis of severe asthma [47]. MBP-stimulated normal human bronchial epithelial cells have elevated expression levels of endothelin-1, transforming growth factor (TGF)-α, TGF-β, platelet-derived growth factor, matrix metallopeptidase 9, and fibronectin, which suggests that MBP affects the composition of the extracellular matrix and turnover of airway epithelium [48]. MBP was also suggested to enhance bronchoconstriction in asthma through blocking muscarinic M2 receptors, followed by eliminating the negative feedback and increasing the release of acetylcholine [49].
Eosinophil-derived neurotoxin
The RNase superfamily member EDN, encoded in humans by the gene RNASE2, is the second-most abundant protein in the human eosinophil proteome out of the 7,086 proteins identified by proteomics of peripheral blood eosinophils [50]. EDN can be isolated not only from eosinophils but also from neutrophils as well as the liver, spleen, kidney, and urine [38,51,52,53]. In neutrophils, EDN is present in the neutrophilic granules, as demonstrated by immunoelectron microscopy [38]. EDN has limited toxicity for helminth parasites compared with MBP and ECP [34,54], but this protein is active against RNA viruses. Additionally, EDN is 100-fold more ribonucleolytically active compared with ECP [55]. Intrathecal injection of EDN or ECP into rabbits causes the death of cerebellar Purkinje fibers, which is known as the Gordon phenomenon [56]. A recent report has shown that EDN can act as an alarmin, is involved in the activation of dendritic cells through the TLR2–MyD88 signaling pathway, and activates the type-2 immune response [57].
Eosinophil cationic protein
ECP, encoded in humans by the gene RNASE3, also belongs to the RNase superfamily. Compared with EDN, ECP is more cationic and more toxic to bacteria [58]. ECP can destabilize bacterial lipid bilayers and neutralize bacterial lipopolysaccharide, which contribute to the toxicity of ECP to bacteria [59,60]. However, ECP has 125 times lower RNase activity compared with EDN [61]. Regarding the toxicity of ECP to Schistosoma mansoni, electron microscopy observation revealed that in ECP-treated S. mansoni, blebs were formed on the surface of the parasite and the surface of the parasite was ruptured [62]. The toxicity to helminth parasites of ECP is equivalent to the effect of MBP, but the effect of ECP is slower than that of MBP [54]. Like MBP, ECP mediates histamine release in basophils and mast cells [63]. ECP increases the activation of kallikrein, enhances factor XII, and shortens the blood coagulation time [64]. ECP is also involved in fibrinolysis via plasminogen enhancement [65]. Additionally, ECP inhibits microbial activity by forming amyloid-like aggregates on bacterial surfaces [66]. In a recent report, ECP and EDN both induced the expression of matrix metalloproteinase 9 in keratinocytes and triggered keratinocyte apoptosis, which suggests their potential as therapeutic targets for bullous pemphigoid [67].
Eosinophil peroxidase
EPO is highly cationic, heme-containing oxidoreductase that is similar to myeloperoxidase (MPO) in neutrophils [68]. Although EPO and MPO have 68.3% of the same amino acids [69], there are some differences in their functions; for example, EPO binds to antineutrophil cytoplasmic antibodies and is involved in renal fibrosis [70,71]. EPO is taken up by neutrophils, basophils, and mast cells, and EPO has a higher affinity for neutrophils compared with MPO [72,73]. By acting as an oxidoreductase, halides are coactivated with reactive oxygen species to produce hypochlorous acid, which is highly toxic to helminth parasites and bacteria [74,75] as well as toxic to tumor cells [76]. Hypothiocyanous acid (HOSCN) is produced by the oxidation of EPO and induces tissue factor activation, which suggests its involvement in thrombosis. HOSCN also activates the proinflammatory p65/p50 nuclear factor-κB pathway [77]. EPO binds to the surface of microorganisms, thereby facilitating macrophage phagocytosis even in microorganisms that are resistant to macrophage destruction [78]. EPO is associated with mucus plugging of the airways in asthma [79]. Similar to MBP and ECP, EPO induces mast cell degranulation [80]. EPO also inactivates leukotrienes B4, C4, and D4 [81].
Galectin-10 (Charcot-Leyden crystal protein)
Galectin-10 is a cytoplasmic protein that belongs to S-lectin family and the fifth most abundant protein in eosinophils [50]. It was originally recognized as Charcot-Leyden crystal (CLC) protein and later named galectin-10 because of its carbohydrate-binding domain, which is similar to those of other galectin family members. Galectin-10 is expressed predominantly on eosinophils but is also present on macrophages, basophils, and CD4+CD25+ regulatory T cells [82]. In unstimulated eosinophils, galectin-10 is localized in the peripheral cytoplasm [83]. During the process of EETosis, galectin-10 can redistribute in the cytoplasm and form CLCs intracellularly [84]. EETosis-mediated plasma membrane disintegration causes the extracellular release of galectin-10, resulting in the extracellular formation of CLCs [84]. Recent reports indicate that CLCs promote type-2 immunity [85] and also neutrophilic inflammation [86]. The full function of galectin-10 is still unclear, but reports suggest that this protein regulates the dynamic palmitoylation cycle [87] and is involved in vesicular transport systems and granulogenesis [88].
Histones and double-stranded DNA
EETosis releases eosinophil extracellular traps, which are composed mainly of histones and double-stranded DNA, that is, chromatin fiber. Eosinophil extracellular traps are effective at trapping microbial pathogens because of their net-like structure [89]. A proteome analysis of human eosinophils indicated that histones ranked 3rd, 4th, 6th, and 15th in abundance among the top 15 proteins [50]. The cytotoxic effects to bacteria of histones have been known for more than 50 years [90]. Histones promote cell apoptosis [91,92] and are involved in inflammation by stimulating Toll-like receptor signaling pathways [93,94,95] or NLRP3 inflammasomes [96,97]. Histones have also been recognized as inducers of the blood coagulation system [98,99,100,101]. Extracellular double-stranded DNA is known to cause vascular damage [102,103] and thrombosis, and it can stimulate autoimmune responses, such as systemic lupus erythematosus [104,105,106].
Other cytokines and lipid mediators
Eosinophils are a rich source of cysteinyl leukotrienes (cysLTs). Leukotriene C4 and its metabolites, leukotriene D4 and leukotriene E4, have varied roles in mediating eosinophilic disorders, including host defense against parasites and allergic inflammation. CysLTs can activate eosinophils in an autocrine manner (they prolong survival), and induce reactive oxygen species production and EDN release from eosinophils [107,108]. CysLTs are also involved in eosinophil differentiation and maturation in combination with IL-5 [109]. Additionally, extracellular eosinophil granules can release ECP in response to cysLT stimulation via cysLT receptors expressed on the granule membrane [110].
Eosinophils store a wide array of cytokines, chemokines, and growth factors, including IL-4 [111,112], granulocyte macrophage colony-stimulating factor (GM-CSF) [113,114,115], and TGF-β [116,117,118]. IL-4 induces eosinophil migration into tissues via the expression of adhesion molecule vascular cell adhesion molecule-1 on endothelial cells [119]. Various stimuli, including calcium ionophore [113,114] and fibronectin [115], can induce GM-CSF production in eosinophils. GM-CSF is involved in the type-2 response in allergic airway inflammation through activating dendritic cells and enhancing eosinophil survival in an autocrine manner [120,121].
PERIPHERAL VERSUS TISSUE EOSINOPHILIA: LESSONS FROM ACUTE EOSINOPHILIC PNEUMONIA
Peripheral blood eosinophilia can assist in the diagnosis of eosinophilic inflammatory diseases, including eosinophilic pneumonia. Unlike the diagnostic criteria for chronic eosinophilic pneumonia with peripheral blood eosinophilia, the diagnostic criteria for acute eosinophilic pneumonia (AEP) do not require peripheral blood eosinophilia [122]. AEP is caused mainly by inhalational exposure, such as cigarette smoke, including electronic cigarettes or heated tobacco [123], and develops acutely along with respiratory failure. Peripheral blood eosinophilia may be absent at the onset of AEP, especially in smoking-related AEP [124].
We experienced a case in which bacterial pneumonia was suspected because of the patient's neutrophilia, and the results of a bronchoalveolar lavage led to the diagnosis of AEP. A 21-year-old woman had fever and dyspnea 1 week after smoking initiation. Upon hospitalization, she was febrile (39.6°C) and tachypneic with a reduced peripheral oxygen saturation (91%). Her arterial blood gases under room air were: pH, 7.44; PaO2, 55 mmHg; and PaCO2, 30 mmHg. Her chest x-ray showed bilateral ground grass attenuations mixed with consolidations and a slight pleural effusion.
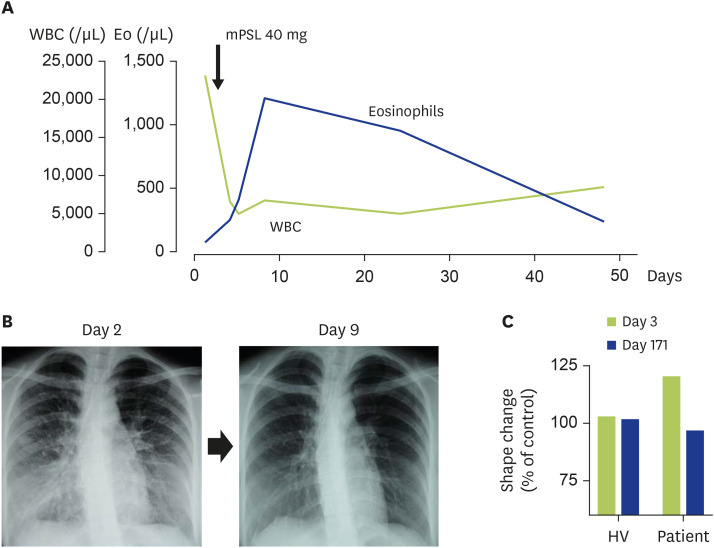
The laboratory test revealed a white blood cell count of 23,400 cells/µL with neutrophilia (95.8%, 22,417 cells/µL). Her serum C-reactive protein level was 8.2 mg/dL (normal, <0.3 mg/dL), IL-5 level was 1,012 pg/mL (normal, <3.9 pg/mL), and ECP level was 68.9 µg/L (normal, <14.9 µg/L), and her peripheral blood eosinophil count was only 94 cells/µL. Conversely, a marked elevation of eosinophils was observed in her bronchoalveolar lavage fluid (71% eosinophils in the differential cell count), leading to the diagnosis of AEP. A single administration of methylprednisolone (40 mg) and the cessation of cigarette smoking dramatically improved the symptoms of AEP. Notably, in parallel with the patient's improvement of bilateral pulmonary infiltration, her peripheral eosinophil counts reached up to 1,250 cells/µL on day 9 (6 days after methylprednisolone administration) (Fig. 1A, B).
Fig. 1. Clinical course of a case with AEP.
(A) Dynamics of blood eosinophil counts. Values indicate the peripheral white blood cell (WBC) counts (green line) and eosinophil (Eo) counts (blue line). On day 2, the patient was treated with an intravenous administration of methylprednisolone (mPSL). (B) Chest x-ray on day 2 (before performing a bronchoalveolar lavage and mPSL administration) and day 9 (6 days after treatment). (C) Response of eosinophils to cigarette smoke extract (CSE). CSE was prepared as described by a previous report [161]. Peripheral whole blood cells stimulated with CSE for 10 minutes and red blood cells were lysed using BD FACS Lysing Solution (BD Biosciences, San Jose, CA, USA). The eosinophil shape change induced by CSE was evaluated using a FACScan flow cytometer (BD Biosciences). Values shown are the % of control buffer. HV, healthy volunteer.
Before or during eosinophil movement, a cellular shape change related to migration is necessary [2]. We conducted an ex vivo eosinophil shape change assay by using flow cytometric measurement of autofluorescence/forward scatter on cells stimulated with cigarette smoke extract [125]. A clear shape change was induced in the patient's eosinophils at the onset, and such changes were not reproduced in cells taken from the patient after the resolution of AEP or in eosinophils from a normal donor (Fig. 1C). Thus, it can be speculated that eosinophils have the potential to accumulate in the lung at the onset of AEP.
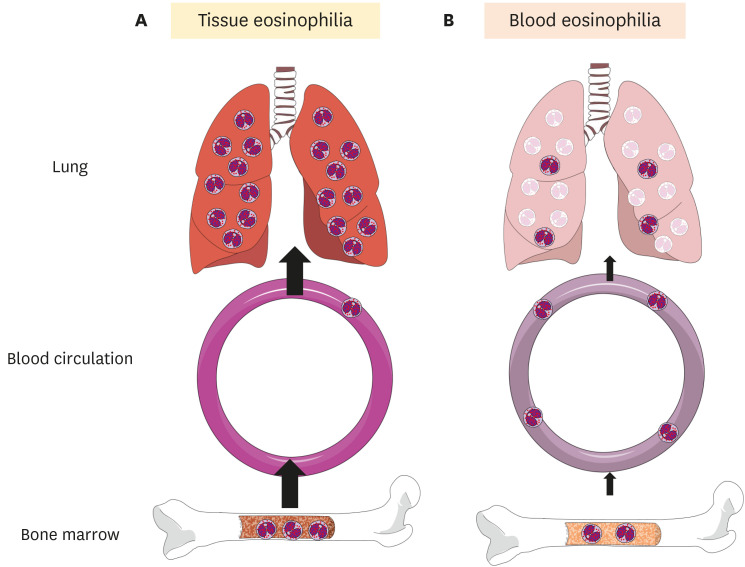
IL-5 plays a critical role in an eosinophilopoiesis, chemokinesis/chemotaxis, integrin activation, and survival prolongation via apoptosis inhibition [6]. In our case, the initially elevated serum IL-5 level was associated with AEP disease severity, despite the peripheral blood eosinophil counts being in the normal range. Interestingly, serum IL-5 levels have been shown to inversely correlate with peripheral blood eosinophil counts in patients in the initial state of AEP [126]. This is likely caused by the rapid migration of blood eosinophils to the lungs, as illustrated in Fig. 2A. Notably, the pathological condition of asthma was recreated in lung-specific IL-5 transgenic mice but not in “systemic” IL-5 transgenic mice [6]. Tissue-specific overproduction of IL-5 might play an important role in the pathogenesis of AEP by recruiting eosinophils from the peripheral blood into the lungs.
Fig. 2. Schematic of tissue and blood eosinophilia in acute eosinophilic pneumonia (AEP).
(A) At the onset of AEP, there is a rapid recruitment of blood eosinophils into the lung, resulting in a normal-range blood eosinophil count. (B) After treatment with systemic steroids, the eosinophilic lung inflammation is resolved, mainly through the induction of eosinophil apoptosis in the lungs. The decreased recruitment of blood eosinophils into the lung might result in the retention of eosinophils in circulation, leading to a transient blood eosinophilia.
In patients with atopic asthma, sputum eosinophil counts and serum IL-5 levels increase after allergen inhalation, whereas blood eosinophil counts decrease for 12 hours [127,128]. The intravascular residence time of radiolabeled eosinophils in healthy volunteers is approximately 25 hours, although it can be 1.5 hours in a patient with tissue eosinophilic inflammation [129,130]. Accumulated eosinophils might directly induce eosinophilia through the production of eosinophil chemoattractants, such as leukotriene B4 [131] and C-C chemokine ligand 4 [132]. These data suggest that a dynamic shift of circulating eosinophils into the tissue can occur.
Transient blood eosinophilia after systemic corticosteroid administration is another interesting feature of the present case. This phenomenon has been reported previously; serum IL-5 rapidly falls into the normal range within 10 days [126], whereas peripheral blood eosinophil counts increase with radiographic and clinical resolution during the following days [126,133,134].
Corticosteroids have various potent anti-inflammatory effects on allergic inflammation. They can induce eosinophil apoptosis directly as well as can indirectly, by inhibiting the production of survival factors including IL-5 [135,136]. In addition, corticosteroids enhance the phagocytic capacity of macrophages and airway epithelial cells [136,137]. Apoptotic eosinophils are intact when they are recognized and engulfed by phagocytes, so they do not induce inflammation [138]. As illustrated in Fig. 2B, after the treatment of AEP with a systemic steroid, eosinophilic lung inflammation is resolved, likely through the clearance of apoptotic eosinophils in the lungs. It is also conceivable that the decreased recruitment of blood eosinophils into the lungs results in a transient blood eosinophilia.
The mismatch of circulating and tissue eosinophilia is not limited to AEP. For instance, we have also experienced a case in which a patient with GM-CSF-producing lung cancer showed no clinical manifestations or evidence of tissue eosinophilia despite having marked blood eosinophilia (>30,000 cells/µL) [139]. Dupilumab, an anti-IL-4Ra antibody that blocks both IL-4 and IL-13 signaling, can trigger eosinophilia without the presentation of clinical signs of organ involvement owing to eosinophil infiltration [140]. The mechanisms underlying dupilumab-induced eosinophilia remain unknown, but it has been hypothesized that dupilumab blocks the migration of eosinophils into tissue without blocking eosinophil production in the bone marrow [141]. Hypereosinophilia is defined by a peripheral blood absolute eosinophil count of greater than 1,500 cells/µL that may not be associated with tissue damage [142]. In addition to patients with hypereosinophilic syndrome, who typically require treatment to prevent disease progression, there are patients with unexplained persistent asymptomatic hypereosinophilia. Additionally, clinical manifestation related to eosinophilic inflammation is uncommon in patients with familial eosinophilia [143]. Thus, the circulating eosinophil count does not always reflect tissue eosinophilia and vice versa.
“EOSINOPHILIC” TISSUE IN THE ABSENCE OF EOSINOPHILS
Tissue hypereosinophilia can be defined as tissue with a percentage of eosinophils that exceeds 20% of all nucleated cells in the bone marrow or tissue infiltration that is deemed extensive by a pathologist [11,144]. However, historical studies with immunostaining for eosinophil granule proteins have revealed the extracellular deposition of granule proteins coincident with pathological conditions, even in the absence of a significant eosinophil infiltrate. Frigas and Gleich [145] described the lung tissue specimens from autopsy cases who died of asthma as follows: “some eosinophils were intact, others were partially degranulated and surrounded by their extruded granules, and others were totally disrupted and unrecognizable by hematoxylin and eosin stain.” In atopic patients challenged with an intradermal injection of allergen, MBP and EDN were extensively deposited throughout the dermis in the late-phase reaction [146]. Tissue deposition of granule protein in the absence of eosinophil accumulation has also been reported by Gleich et al. in cases of Hodgkin's disease [147], parasite infection [148,149], chronic urticaria [150], atopic dermatitis [151], and endomyocardial disease [152]. Several studies have indicated that granule protein deposition, rather than intact eosinophils, is associated with tissue remodeling [153,154]. Given the toxicity of eosinophil granule proteins, histologic evidence of extracellular granule protein deposition in the tissue might be a more appropriate marker for inflammation than tissue eosinophilia.
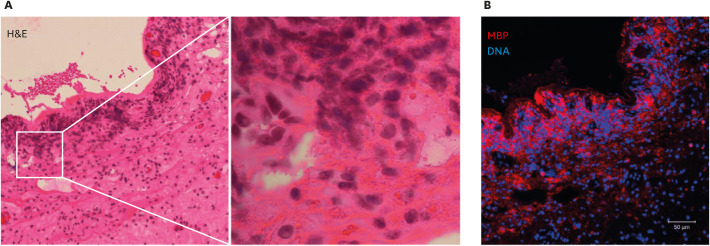
Our group has studied hundreds of tissues from patients with allergic or eosinophilic diseases [26,29,83,84,155,156,157,158,159,160]. In our tissue immunostaining experience, like Gleich et al., we have observed that the extracellular deposition of MBP is not disease-specific, but it is closely associated with tissue damage and the presence of eosinophil cytolysis. Fig. 3 shows a typical example: surgically obtained nasal polyps from a case of chronic rhinosinusitis with nasal polyps (eosinophilic chronic rhinosinusitis). Hematoxylin and eosin staining revealed that the accumulated eosinophils were cytolytic, showing extracellular cell-free granules and chromatolysis and/or a loss of nuclear envelope. Immunostaining indicated a massive deposition of extracellular MBP that is consistent with the presence of cytolytic eosinophils. These cytolytic eosinophils were ultrastructurally identical to EETosis induced by various stimuli in vitro [26,83].
Fig. 3. Cytolytic eosinophils in a nasal polyp obtained from a case of chronic rhinosinusitis with nasal polyps (eosinophilic chronic rhinosinusitis).
(A) Hematoxylin and eosin (H&E) staining of a nasal polyp, showing a loss of epithelium and inflammatory cell infiltration in submucosal tissue. The boxed area in the left panel is shown magnified in the right panel. Accumulated cells showed cytolysis and a loss of nuclear shape (chromatolysis). Eosinophilic extracellular granules were also noted. (B) A serial section of the tissue shown in panel A was immunostained for major basic protein (MBP) (red) and counterstained for DNA (blue). Image was obtained with a Carl Zeiss LSM780 confocal microscope (×20). The massive deposition of extracellular MBP visible is consistent with the presence of cytolytic eosinophils.
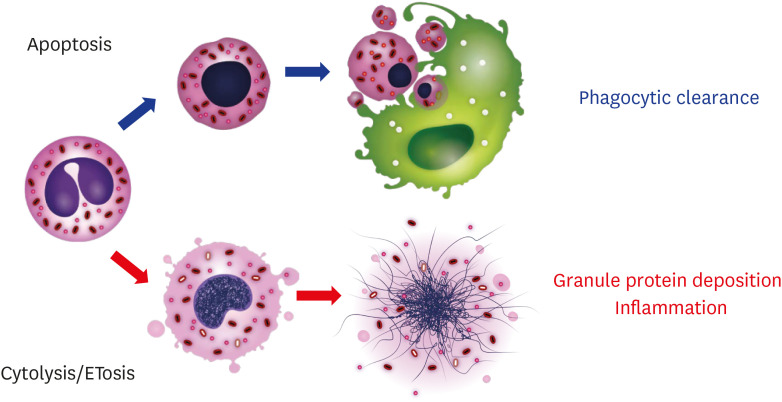
Apoptosis facilitates the ingestion of intact eosinophils without a disgorgement of their toxic contents, and this process is necessary for the normal resolution of inflammation [138]. Impaired phagocytic clearance (efferocytosis) of lytic eosinophils is a critical feature of persistent inflammation. Notably, efferocytosis by phagocytic cells might not be applicable for ETotic cells, because of their rapid cell death process (0.5–3 hours) and lack of find-me signal exposure before cell lysis [25,26]. Therefore, eosinophil cell fate within tissues might have completely different consequences (Fig. 4).
Fig. 4. Eosinophil cell fates and their consequences.
Eosinophils apoptosis can be caused by many factors, including aging, a loss of survival factors, corticosteroids, and anti-interleukin (IL)-5/anti-IL-5 receptor antibodies. Apoptotic eosinophils, typically with nuclear and cytoplasmic condensation, are phagocytosed without the induction of inflammation. Alternately, eosinophils can undergo ETosis upon activation, such as by an immunoglobulin-coated surface, pathogens, or platelet-activating factor with IL-5. Rapid cytolysis without the expression of a “find-me” signal results in the tissue deposition of the cell's total intracellular contents and prolonged inflammation.
CONCLUSIONS
The presence of eosinophils is an important feature of type-2 inflammation, but clinical observations have indicated that eosinophil-mediated inflammation is not always coincident with an increase in eosinophil counts. As shown in the case of AEP, there are various examples of mismatch between the circulating eosinophil count and clinical manifestation. As stated above, considerable evidence has indicated that the marked deposition of eosinophil granule proteins in tissue is associated with tissue damage and remodeling. The most important feature of eosinophils as end-stage effector cells is their activation to release toxic cellular contents. In this context, eosinophils can be innocent bystanders without the secretion of their bioactive mediators.
EETosis is now considered to be a major mechanism of cytolytic degranulation. This process is not only a total cell degranulation but also a release of cytoplasmic and nuclear contents, including DNA and histones that act as alarmins. Although the maintenance of tissue eosinophils is tightly related to the ability of the cell and the microenvironment to maintain an appropriate balance between survival and active cell death pathways, it is not yet fully understood. Further study will lead to a better understanding of eosinophil-mediated inflammation and its treatment.
ACKNOWLEDGEMENTS
The authors are grateful to Noriko Tan for technical assistance and Satomi Misawa for outstanding assistance in drawing the figures. They also thank Katie Oakley, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript. For all clinical samples, written informed consent was obtained from all participants in accordance with the Declaration of Helsinki and institutional review board-approved protocols.
Footnotes
Conflict of interest: MF received grant support from GlaxoSmithKline Japan Research Grants 2018; PA has received research support and consultancy fees from and has been on advisory boards for AstraZeneca and GlaxoSmithKline; SU received honoraria for lectures from AstraZeneca and GlaxoSmithKline as well as grant support from AstraZeneca, Novartis, and Maruho. The rest of the authors have no conflicts of interest.
- Conceptualization: Shigeharu Ueki, Yui Miyabe, Mineyo Fukuchi, Yoshiki Kobayashi.
- Investigation: Yui Miyabe, Mineyo Fukuchi, Yoshiki Kobayashi.
- Project administration: Akiko Saga, Yuki Moritoki, Tomoo Saga, Shigeharu Ueki.
- Writing - original draft: Yui Miyabe, Mineyo Fukuchi, Yoshiki Kobayashi, Shigeharu Ueki.
- Writing - review & editing: Yui Miyabe, Mineyo Fukuchi, Praveen Akuthota, Akiko Saga, Yuki Moritoki, Tomoo Saga,Shigeharu Ueki.
References
- 1.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 2.Ueki S, Kihara J, Kato H, Ito W, Takeda M, Kobayashi Y, Kayaba H, Chihara J. Soluble vascular cell adhesion molecule-1 induces human eosinophil migration. Allergy. 2009;64:718–724. doi: 10.1111/j.1398-9995.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 3.Gouon-Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515–4521. doi: 10.1210/endo.142.10.8459. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki M, Konno Y, Kobayashi Y, Takeda M, Itoga M, Moritoki Y, Oyamada H, Kayaba H, Chihara J, Ueki S. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils. Immunol Lett. 2014;160:72–78. doi: 10.1016/j.imlet.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Weller PF, Spencer LA. Functions of tissue-resident eosinophils. Nat Rev Immunol. 2017;17:746–760. doi: 10.1038/nri.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69:178–186. doi: 10.1016/j.alit.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Hambleton K, Pavord ID. What can we learn from blood granulocyte patterns in patients with asthma? Eur Respir J. 2016;48:976–978. doi: 10.1183/13993003.01567-2016. [DOI] [PubMed] [Google Scholar]
- 8.Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146:1–7. doi: 10.1016/j.jaci.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, Rosenstreich D, Ramesh M. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract. 2021;9:1152–62.e3. doi: 10.1016/j.jaip.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne) 2017;4:101. doi: 10.3389/fmed.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, Hellmann A, Metzgeroth G, Leiferman KM, Arock M, Butterfield JH, Sperr WR, Sotlar K, Vandenberghe P, Haferlach T, Simon HU, Reiter A, Gleich GJ. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607–12.e9. doi: 10.1016/j.jaci.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophils to human health and disease. Annu Rev Pathol. 2020;15:179–209. doi: 10.1146/annurev-pathmechdis-012419-032756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asano K, Ueki S, Tamari M, Imoto Y, Fujieda S, Taniguchi M. Adult-onset eosinophilic airway diseases. Allergy. 2020;75:3087–3099. doi: 10.1111/all.14620. [DOI] [PubMed] [Google Scholar]
- 14.Uller L, Persson CG, Källström L, Erjefält JS. Lung tissue eosinophils may be cleared through luminal entry rather than apoptosis: effects of steroid treatment. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1948–1956. doi: 10.1164/ajrccm.164.10.2011135. [DOI] [PubMed] [Google Scholar]
- 15.Venge P, Håkansson L, Peterson CG. Eosinophil activation in allergic disease. Int Arch Allergy Appl Immunol. 1987;82:333–337. doi: 10.1159/000234219. [DOI] [PubMed] [Google Scholar]
- 16.Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–38. doi: 10.1016/j.coi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Ueki S, Ohta N, Takeda M, Konno Y, Hirokawa M. Eosinophilic otitis media: the aftermath of eosinophil extracellular trap cell death. Curr Allergy Asthma Rep. 2017;17:33. doi: 10.1007/s11882-017-0702-5. [DOI] [PubMed] [Google Scholar]
- 18.Melo RCN, Weller PF. Contemporary understanding of the secretory granules in human eosinophils. J Leukoc Biol. 2018;104:85–93. doi: 10.1002/JLB.3MR1217-476R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer LA, Bonjour K, Melo RC, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo RC, Weller PF. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol. 2010;25:1341–1354. doi: 10.14670/hh-25.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1:pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 24.Guimarães-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH, Saraiva EM. ETosis: a microbicidal mechanism beyond cell death. J Parasitol Res. 2012;2012:929743. doi: 10.1155/2012/929743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF. Eosinophil ETosis and DNA traps: a new look at eosinophilic inflammation. Curr Allergy Asthma Rep. 2016;16:54. doi: 10.1007/s11882-016-0634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, Weller PF. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J. 2012;26:2084–2093. doi: 10.1096/fj.11-200246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018;141:571–85.e7. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DM, Lewis JC, Loegering DA, Gleich GJ. Localization of the guinea pig eosinophil major basic protein to the core of the granule. J Cell Biol. 1978;77:702–713. doi: 10.1083/jcb.77.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackerman SJ, Loegering DA, Venge P, Olsson I, Harley JB, Fauci AS, Gleich GJ. Distinctive cationic proteins of the human eosinophil granule: major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin. J Immunol. 1983;131:2977–2982. [PubMed] [Google Scholar]
- 33.Lewis DM, Loegering DA, Gleich GJ. Isolation and partial characterization of a major basic protein from rat eosinophil granules. Proc Soc Exp Biol Med. 1976;152:512–515. doi: 10.3181/00379727-152-39429. [DOI] [PubMed] [Google Scholar]
- 34.Hamann KJ, Gleich GJ, Checkel JL, Loegering DA, McCall JW, Barker RL. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J Immunol. 1990;144:3166–3173. [PubMed] [Google Scholar]
- 35.Plager DA, Loegering DA, Checkel JL, Tang J, Kephart GM, Caffes PL, Adolphson CR, Ohnuki LE, Gleich GJ. Major basic protein homolog (MBP2): a specific human eosinophil marker. J Immunol. 2006;177:7340–7345. doi: 10.4049/jimmunol.177.10.7340. [DOI] [PubMed] [Google Scholar]
- 36.Nair P, Ochkur SI, Protheroe C, Radford K, Efthimiadis A, Lee NA, Lee JJ. Eosinophil peroxidase in sputum represents a unique biomarker of airway eosinophilia. Allergy. 2013;68:1177–1184. doi: 10.1111/all.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima T, Matsumoto K, Suto H, Tanaka K, Ebisawa M, Tomita H, Yuki K, Katsunuma T, Akasawa A, Hashida R, Sugita Y, Ogawa H, Ra C, Saito H. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood. 2001;98:1127–1134. doi: 10.1182/blood.v98.4.1127. [DOI] [PubMed] [Google Scholar]
- 38.Sur S, Glitz DG, Kita H, Kujawa SM, Peterson EA, Weiler DA, Kephart GM, Wagner JM, George TJ, Gleich GJ, Leiferman KM. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J Leukoc Biol. 1998;63:715–722. doi: 10.1002/jlb.63.6.715. [DOI] [PubMed] [Google Scholar]
- 39.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleich GJ, Loegering DA, Mann KG, Maldonado JE. Comparative properties of the Charcot-Leyden crystal protein and the major basic protein from human eosinophils. J Clin Invest. 1976;57:633–640. doi: 10.1172/JCI108319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989;142:4428–4434. [PubMed] [Google Scholar]
- 42.O'Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med. 1983;157:1981–1991. doi: 10.1084/jem.157.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, Kuroda K, Nunomura S, Hayama K, Terui T, Ra C, Okayama Y. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134:622–33.e9. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Rohrbach MS, Wheatley CL, Slifman NR, Gleich GJ. Activation of platelets by eosinophil granule proteins. J Exp Med. 1990;172:1271–1274. doi: 10.1084/jem.172.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kita H, Abu-Ghazaleh RI, Sur S, Gleich GJ. Eosinophil major basic protein induces degranulation and IL-8 production by human eosinophils. J Immunol. 1995;154:4749–4758. [PubMed] [Google Scholar]
- 46.Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925–2927. [PubMed] [Google Scholar]
- 47.Hastie AT, Loegering DA, Gleich GJ, Kueppers F. The effect of purified human eosinophil major basic protein on mammalian ciliary activity. Am Rev Respir Dis. 1987;135:848–853. doi: 10.1164/arrd.1987.135.4.848. [DOI] [PubMed] [Google Scholar]
- 48.Pégorier S, Wagner LA, Gleich GJ, Pretolani M. Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol. 2006;177:4861–4869. doi: 10.4049/jimmunol.177.7.4861. [DOI] [PubMed] [Google Scholar]
- 49.Jacoby DB, Gleich GJ, Fryer AD. Human eosinophil major basic protein is an endogenous allosteric antagonist at the inhibitory muscarinic M2 receptor. J Clin Invest. 1993;91:1314–1318. doi: 10.1172/JCI116331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkerson EM, Johansson MW, Hebert AS, Westphall MS, Mathur SK, Jarjour NN, Schwantes EA, Mosher DF, Coon JJ. The peripheral blood eosinophil proteome. J Proteome Res. 2016;15:1524–1533. doi: 10.1021/acs.jproteome.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorrentino S, Tucker GK, Glitz DG. Purification and characterization of a ribonuclease from human liver. J Biol Chem. 1988;263:16125–16131. [PubMed] [Google Scholar]
- 52.Yasuda T, Mizuta K, Sato W, Kishi K. Purification and characterization of a ribonuclease from human spleen. Immunological and enzymological comparison with nonsecretory ribonuclease from human urine. Eur J Biochem. 1990;191:523–529. doi: 10.1111/j.1432-1033.1990.tb19152.x. [DOI] [PubMed] [Google Scholar]
- 53.Mizuta K, Awazu S, Yasuda T, Kishi K. Purification and characterization of three ribonucleases from human kidney: comparison with urine ribonucleases. Arch Biochem Biophys. 1990;281:144–151. doi: 10.1016/0003-9861(90)90424-w. [DOI] [PubMed] [Google Scholar]
- 54.Hamann KJ, Barker RL, Loegering DA, Gleich GJ. Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. J Parasitol. 1987;73:523–529. [PubMed] [Google Scholar]
- 55.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 56.Gleich GJ, Loegering DA, Bell MP, Checkel JL, Ackerman SJ, McKean DJ. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci U S A. 1986;83:3146–3150. doi: 10.1073/pnas.83.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenberg HF, Ackerman SJ, Tenen DG. Human eosinophil cationic protein. Molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J Exp Med. 1989;170:163–176. doi: 10.1084/jem.170.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torrent M, Navarro S, Moussaoui M, Nogués MV, Boix E. Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry. 2008;47:3544–3555. doi: 10.1021/bi702065b. [DOI] [PubMed] [Google Scholar]
- 60.Pulido D, Garcia-Mayoral MF, Moussaoui M, Velázquez D, Torrent M, Bruix M, Boix E. Structural basis for endotoxin neutralization by the eosinophil cationic protein. FEBS J. 2016;283:4176–4191. doi: 10.1111/febs.13915. [DOI] [PubMed] [Google Scholar]
- 61.Gullberg U, Widegren B, Arnason U, Egesten A, Olsson I. The cytotoxic eosinophil cationic protein (ECP) has ribonuclease activity. Biochem Biophys Res Commun. 1986;139:1239–1242. doi: 10.1016/s0006-291x(86)80310-2. [DOI] [PubMed] [Google Scholar]
- 62.McLaren DJ, McKean JR, Olsson I, Venges P, Kay AB. Morphological studies on the killing of schistosomula of Schistosoma mansoni by human eosinophil and neutrophil cationic proteins in vitro. Parasite Immunol. 1981;3:359–373. doi: 10.1111/j.1365-3024.1981.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 63.Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol. 1984;133:2180–2185. [PubMed] [Google Scholar]
- 64.Venge P, Dahl R, Hällgren R. Enhancement of factor XII dependent reactions by eosinophil cationic protein. Thromb Res. 1979;14:641–649. doi: 10.1016/0049-3848(79)90119-1. [DOI] [PubMed] [Google Scholar]
- 65.Dahl R, Venge P. Enhancement of urokinase-induced plasminogen activation by the cationic protein of human eosinophil granulocytes. Thromb Res. 1979;14:599–608. doi: 10.1016/0049-3848(79)90115-4. [DOI] [PubMed] [Google Scholar]
- 66.Torrent M, Pulido D, Nogués MV, Boix E. Exploring new biological functions of amyloids: bacteria cell agglutination mediated by host protein aggregation. PLoS Pathog. 2012;8:e1003005. doi: 10.1371/journal.ppat.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amber KT, Chernyavsky A, Agnoletti AF, Cozzani E, Grando SA. Mechanisms of pathogenic effects of eosinophil cationic protein and eosinophil-derived neurotoxin on human keratinocytes. Exp Dermatol. 2018;27:1322–1327. doi: 10.1111/exd.13782. [DOI] [PubMed] [Google Scholar]
- 68.Wang JG, Mahmud SA, Nguyen J, Slungaard A. Thiocyanate-dependent induction of endothelial cell adhesion molecule expression by phagocyte peroxidases: a novel HOSCN-specific oxidant mechanism to amplify inflammation. J Immunol. 2006;177:8714–8722. doi: 10.4049/jimmunol.177.12.8714. [DOI] [PubMed] [Google Scholar]
- 69.Ten RM, Pease LR, McKean DJ, Bell MP, Gleich GJ. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan S, Salapow MA, Breen R, Broide DH. Eosinophil peroxidase differs from neutrophil myeloperoxidase in its ability to bind antineutrophil cytoplasmic antibodies reactive with myeloperoxidase. Int Arch Allergy Immunol. 1994;105:150–154. doi: 10.1159/000236817. [DOI] [PubMed] [Google Scholar]
- 71.Colon S, Luan H, Liu Y, Meyer C, Gewin L, Bhave G. Peroxidasin and eosinophil peroxidase, but not myeloperoxidase, contribute to renal fibrosis in the murine unilateral ureteral obstruction model. Am J Physiol Renal Physiol. 2019;316:F360–F371. doi: 10.1152/ajprenal.00291.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zabucchi G, Menegazzi R, Soranzo MR, Patriarca P. Uptake of human eosinophil peroxidase by human neutrophils. Am J Pathol. 1986;124:510–518. [PMC free article] [PubMed] [Google Scholar]
- 73.Dvorak AM, Klebanoff SJ, Henderson WR, Monahan RA, Pyne K, Galli SJ. Vesicular uptake of eosinophil peroxidase by guinea pig basophils and by cloned mouse mast cells and granule-containing lymphoid cells. Am J Pathol. 1985;118:425–438. [PMC free article] [PubMed] [Google Scholar]
- 74.Jong EC, Chi EY, Klebanoff SJ. Human neutrophil-mediated killing of schistosomula of Schistosoma mansoni: augmentation by schistosomal binding of eosinophil peroxidase. Am J Trop Med Hyg. 1984;33:104–115. doi: 10.4269/ajtmh.1984.33.104. [DOI] [PubMed] [Google Scholar]
- 75.Migler R, DeChatelet LR, Bass DA. Human eosinophilic peroxidase: role in bactericidal activity. Blood. 1978;51:445–456. [PubMed] [Google Scholar]
- 76.Jong EC, Klebanoff SJ. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol. 1980;124:1949–1953. [PubMed] [Google Scholar]
- 77.Wang JG, Mahmud SA, Thompson JA, Geng JG, Key NS, Slungaard A. The principal eosinophil peroxidase product, HOSCN, is a uniquely potent phagocyte oxidant inducer of endothelial cell tissue factor activity: a potential mechanism for thrombosis in eosinophilic inflammatory states. Blood. 2006;107:558–565. doi: 10.1182/blood-2005-05-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramsey PG, Martin T, Chi E, Klebanoff SJ. Arming of mononuclear phagocytes by eosinophil peroxidase bound to Staphylococcus aureus. J Immunol. 1982;128:415–420. [PubMed] [Google Scholar]
- 79.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, Raymond WW, Lachowicz-Scroggins ME, Di Maio S, Hoffman EA, Castro M, Fain SB, Jarjour NN, Israel E, Levy BD, Erzurum SC, Wenzel SE, Meyers DA, Bleecker ER, Phillips BR, Mauger DT, Gordon ED, Woodruff PG, Peters MC, Fahy JV National Heart Lung and Blood Institute (NHLBI) Severe Asthma Research Program (SARP) Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henderson WR, Chi EY, Klebanoff SJ. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980;152:265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henderson WR, Jörg A, Klebanoff SJ. Eosinophil peroxidase-mediated inactivation of leukotrienes B4, C4, and D4. J Immunol. 1982;128:2609–2613. [PubMed] [Google Scholar]
- 82.Su J. A brief history of Charcot-Leyden Crystal protein/galectin-10 research. Molecules. 2018;23:2931. doi: 10.3390/molecules23112931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Melo RCN, Wang H, Silva TP, Imoto Y, Fujieda S, Fukuchi M, Miyabe Y, Hirokawa M, Ueki S, Weller PF. Galectin-10, the protein that forms Charcot-Leyden crystals, is not stored in granules but resides in the peripheral cytoplasm of human eosinophils. J Leukoc Biol. 2020;108:139–149. doi: 10.1002/JLB.3AB0220-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueki S, Tokunaga T, Melo RCN, Saito H, Honda K, Fukuchi M, Konno Y, Takeda M, Yamamoto Y, Hirokawa M, Fujieda S, Spencer LA, Weller PF. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018;132:2183–2187. doi: 10.1182/blood-2018-04-842260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Persson EK, Verstraete K, Heyndrickx I, Gevaert E, Aegerter H, Percier JM, Deswarte K, Verschueren KHG, Dansercoer A, Gras D, Chanez P, Bachert C, Gonçalves A, Van Gorp H, De Haard H, Blanchetot C, Saunders M, Hammad H, Savvides SN, Lambrecht BN. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science. 2019;364:eaaw4295. doi: 10.1126/science.aaw4295. [DOI] [PubMed] [Google Scholar]
- 86.Gevaert E, Delemarre T, De Volder J, Zhang N, Holtappels G, De Ruyck N, Persson E, Heyndrickx I, Verstraete K, Aegerter H, Nauwynck H, Savvides SN, Lambrecht BN, Bachert C. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J Allergy Clin Immunol. 2020;145:427–30.e4. doi: 10.1016/j.jaci.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 87.Weller PF, Wang H, Melo RCN. The Charcot-Leyden crystal protein revisited-A lysopalmitoylphospholipase and more. J Leukoc Biol. 2020;108:105–112. doi: 10.1002/JLB.3MR0320-319RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grozdanovic MM, Doyle CB, Liu L, Maybruck BT, Kwatia MA, Thiyagarajan N, Acharya KR, Ackerman SJ. Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis. J Allergy Clin Immunol. 2020;146:377–89.e10. doi: 10.1016/j.jaci.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirsch JG. Bactericidal action of histone. J Exp Med. 1958;108:925–944. doi: 10.1084/jem.108.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barrero CA, Perez-Leal O, Aksoy M, Moncada C, Ji R, Lopez Y, Mallilankaraman K, Madesh M, Criner GJ, Kelsen SG, Merali S. Histone 3.3 participates in a self-sustaining cascade of apoptosis that contributes to the progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:673–683. doi: 10.1164/rccm.201302-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilthorpe JD, Oozeer F, Nash J, Calvo M, Bennett DL, Lumsden A, Pini A. Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000 Res. 2013;2:148. doi: 10.12688/f1000research.2-148.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawano H, Ito T, Yamada S, Hashiguchi T, Maruyama I, Hisatomi T, Nakamura M, Sakamoto T. Toxic effects of extracellular histones and their neutralization by vitreous in retinal detachment. Lab Invest. 2014;94:569–585. doi: 10.1038/labinvest.2014.46. [DOI] [PubMed] [Google Scholar]
- 95.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allam R, Darisipudi MN, Tschopp J, Anders HJ. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol. 2013;43:3336–3342. doi: 10.1002/eji.201243224. [DOI] [PubMed] [Google Scholar]
- 97.Huang H, Chen HW, Evankovich J, Yan W, Rosborough BR, Nace GW, Ding Q, Loughran P, Beer-Stolz D, Billiar TR, Esmon CT, Tsung A. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol. 2013;191:2665–2679. doi: 10.4049/jimmunol.1202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 100.Carestia A, Rivadeneyra L, Romaniuk MA, Fondevila C, Negrotto S, Schattner M. Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost. 2013;110:1035–1045. doi: 10.1160/TH13-02-0174. [DOI] [PubMed] [Google Scholar]
- 101.Lam FW, Cruz MA, Leung HC, Parikh KS, Smith CW, Rumbaut RE. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132:69–76. doi: 10.1016/j.thromres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 102.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, Shealy D, Denny MF, Plumas J, Chaperot L, Kretzler M, Bruce AT, Kaplan MJ. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19-73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee E, Robertson T, Smith J, Kilfeather S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am J Respir Crit Care Med. 2000;161:1881–1886. doi: 10.1164/ajrccm.161.6.9907054. [DOI] [PubMed] [Google Scholar]
- 108.Saito K, Nagata M, Kikuchi I, Sakamoto Y. Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via beta2 integrin. Ann Allergy Asthma Immunol. 2004;93:594–600. doi: 10.1016/S1081-1206(10)61269-0. [DOI] [PubMed] [Google Scholar]
- 109.Saito H, Morikawa H, Howie K, Crawford L, Baatjes AJ, Denburg E, Cyr MM, Denburg JA. Effects of a cysteinyl leukotriene receptor antagonist on eosinophil recruitment in experimental allergic rhinitis. Immunology. 2004;113:246–252. doi: 10.1111/j.1365-2567.2004.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol. 2010;125:477–482. doi: 10.1016/j.jaci.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konno Y, Ueki S, Takeda M, Kobayashi Y, Tamaki M, Moritoki Y, Oyamada H, Itoga M, Kayaba H, Omokawa A, Hirokawa M. Functional analysis of free fatty acid receptor GPR120 in human eosinophils: implications in metabolic homeostasis. PLoS One. 2015;10:e0120386. doi: 10.1371/journal.pone.0120386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bandeira-Melo C, Hall JC, Penrose JF, Weller PF. Cysteinyl leukotrienes induce IL-4 release from cord blood-derived human eosinophils. J Allergy Clin Immunol. 2002;109:975–979. doi: 10.1067/mai.2002.124269. [DOI] [PubMed] [Google Scholar]
- 113.Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174:745–748. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moqbel R, Hamid Q, Ying S, Barkans J, Hartnell A, Tsicopoulos A, Wardlaw AJ, Kay AB. Expression of mRNA and immunoreactivity for the granulocyte/macrophage colony-stimulating factor in activated human eosinophils. J Exp Med. 1991;174:749–752. doi: 10.1084/jem.174.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anwar AR, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O'Byrne P, Tamura G, Jordana M, Shirato K. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1996;15:404–409. doi: 10.1165/ajrcmb.15.3.8810646. [DOI] [PubMed] [Google Scholar]
- 117.Minshall EM, Leung DYM, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–333. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 118.Wong DT, Elovic A, Matossian K, Nagura N, McBride J, Chou MY, Gordon JR, Rand TH, Galli SJ, Weller PF. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991;78:2702–2707. [PubMed] [Google Scholar]
- 119.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 120.Yamashita N, Tashimo H, Ishida H, Kaneko F, Nakano J, Kato H, Hirai K, Horiuchi T, Ohta K. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) Cell Immunol. 2002;219:92–97. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 121.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1α controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Akuthota P, Weller PF. Eosinophilic pneumonias. Clin Microbiol Rev. 2012;25:649–660. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakao S. Acute eosinophilic pneumonia and heated tobacco products. Intern Med. 2020;59:2807. doi: 10.2169/internalmedicine.5421-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Giacomi F, Vassallo R, Yi ES, Ryu JH. Acute eosinophilic pneumonia causes, diagnosis, and management. Am J Respir Crit Care Med. 2018;197:728–736. doi: 10.1164/rccm.201710-1967CI. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi Y, Ueki S, Mahemuti G, Chiba T, Oyamada H, Saito N, Kanda A, Kayaba H, Chihara J. Physiological levels of 15-deoxy-Delta12,14-prostaglandin J2 prime eotaxin-induced chemotaxis on human eosinophils through peroxisome proliferator-activated receptor-gamma ligation. J Immunol. 2005;175:5744–5750. doi: 10.4049/jimmunol.175.9.5744. [DOI] [PubMed] [Google Scholar]
- 126.Jhun BW, Kim SJ, Kim K, Lee JE, Hong DJ. Clinical implications of correlation between peripheral eosinophil count and serum levels of IL-5 and tryptase in acute eosinophilic pneumonia. Respir Med. 2014;108:1655–1662. doi: 10.1016/j.rmed.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 127.Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy. 1985;15:411–418. doi: 10.1111/j.1365-2222.1985.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 128.Dorman SC, Sehmi R, Gauvreau GM, Watson RM, Foley R, Jones GL, Denburg JA, Inman MD, O'Byrne PM. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;169:565–572. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- 129.Farahi N, Loutsios C, Peters AM, Condliffe AM, Chilvers ER. Use of technetium-99m-labeled eosinophils to detect active eosinophilic inflammation in humans. Am J Respir Crit Care Med. 2013;188:880–882. doi: 10.1164/rccm.201303-0535LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Farahi N, Singh NR, Heard S, Loutsios C, Summers C, Solanki CK, Solanki K, Balan KK, Ruparelia P, Peters AM, Condliffe AM, Chilvers ER. Use of 111-Indium-labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. 2012;120:4068–4071. doi: 10.1182/blood-2012-07-443424. [DOI] [PubMed] [Google Scholar]
- 131.Patnode ML, Bando JK, Krummel MF, Locksley RM, Rosen SD. Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J Exp Med. 2014;211:1281–1288. doi: 10.1084/jem.20132336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kobayashi Y, Konno Y, Kanda A, Yamada Y, Yasuba H, Sakata Y, Fukuchi M, Tomoda K, Iwai H, Ueki S. Critical role of CCL4 in eosinophil recruitment into the airway. Clin Exp Allergy. 2019;49:853–860. doi: 10.1111/cea.13382. [DOI] [PubMed] [Google Scholar]
- 133.Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68:413–419. doi: 10.1016/j.alit.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 134.Philit F, Etienne-Mastroïanni B, Parrot A, Guérin C, Robert D, Cordier JF. Idiopathic acute eosinophilic pneumonia: a study of 22 patients. Am J Respir Crit Care Med. 2002;166:1235–1239. doi: 10.1164/rccm.2112056. [DOI] [PubMed] [Google Scholar]
- 135.Bentley AM, Hamid Q, Robinson DS, Schotman E, Meng Q, Assoufi B, Kay AB, Durham SR. Prednisolone treatment in asthma. Reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med. 1996;153:551–556. doi: 10.1164/ajrccm.153.2.8564096. [DOI] [PubMed] [Google Scholar]
- 136.Druilhe A, Létuvé S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 137.Felton JM, Lucas CD, Rossi AG, Dransfield I. Eosinophils in the lung - modulating apoptosis and efferocytosis in airway inflammation. Front Immunol. 2014;5:302. doi: 10.3389/fimmu.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stern M, Meagher L, Savill J, Haslett C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J Immunol. 1992;148:3543–3549. [PubMed] [Google Scholar]
- 139.Izumiya Y, Okuda Y, Ueki S, Takeda M, Sato K, Nakayama K. Unusual morphologies of blood eosinophils in GM-CSF-producing lung cancer. QJM. 2021;114:42–44. doi: 10.1093/qjmed/hcaa144. [DOI] [PubMed] [Google Scholar]
- 140.Faiz S, Giovannelli J, Podevin C, Jachiet M, Bouaziz JD, Reguiai Z, Nosbaum A, Lasek A, Ferrier le Bouedec MC, Du Thanh A, Raison-Peyron N, Tetart F, Duval-Modeste AB, Misery L, Aubin F, Dompmartin A, Morice C, Droitcourt C, Soria A, Arnault JP, Delaunay J, Mahé E, Richard MA, Schoeffler A, Lacour JP, Begon E, Walter-Lepage A, Dillies AS, Rappelle-Duruy S, Barete S, Bellon N, Bénéton N, Valois A, Barbarot S, Sénéchal J, Staumont-Sallé D Groupe de Recherche sur l'Eczéma aTopique (GREAT), France. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019;81:143–151. doi: 10.1016/j.jaad.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 141.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, Zhu H, Hamilton JD, Swanson BN, Khan A, Chao J, Staudinger H, Pirozzi G, Antoni C, Amin N, Ruddy M, Akinlade B, Graham NMH, Stahl N, Yancopoulos GD, Teper A. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 142.Gotlib J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017;92:1243–1259. doi: 10.1002/ajh.24880. [DOI] [PubMed] [Google Scholar]
- 143.Prakash Babu S, Chen YK, Bonne-Annee S, Yang J, Maric I, Myers TG, Nutman TB, Klion AD. Dysregulation of interleukin 5 expression in familial eosinophilia. Allergy. 2017;72:1338–1345. doi: 10.1111/all.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwartz JT, Fulkerson PC. An approach to the evaluation of persistent hypereosinophilia in pediatric patients. Front Immunol. 2018;9:1944. doi: 10.3389/fimmu.2018.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986;77:527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- 146.Leiferman KM, Fujisawa T, Gray BH, Gleich GJ. Extracellular deposition of eosinophil and neutrophil granule proteins in the IgE-mediated cutaneous late phase reaction. Lab Invest. 1990;62:579–589. [PubMed] [Google Scholar]
- 147.Butterfield JH, Kephart GM, Banks PM, Gleich GJ. Extracellular deposition of eosinophil granule major basic protein in lymph nodes of patients with Hodgkin's disease. Blood. 1986;68:1250–1256. [PubMed] [Google Scholar]
- 148.Kephart GM, Andrade ZA, Gleich GJ. Localization of eosinophil major basic protein onto eggs of Schistosoma mansoni in human pathologic tissue. Am J Pathol. 1988;133:389–396. [PMC free article] [PubMed] [Google Scholar]
- 149.Kephart GM, Gleich GJ, Connor DH, Gibson DW, Ackerman SJ. Deposition of eosinophil granule major basic protein onto microfilariae of Onchocerca volvulus in the skin of patients treated with diethylcarbamazine. Lab Invest. 1984;50:51–61. [PubMed] [Google Scholar]
- 150.Peters MS, Schroeter AL, Kephart GM, Gleich GJ. Localization of eosinophil granule major basic protein in chronic urticaria. J Invest Dermatol. 1983;81:39–43. doi: 10.1111/1523-1747.ep12538380. [DOI] [PubMed] [Google Scholar]
- 151.Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, Leiferman KM. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J Allergy Clin Immunol. 1997;99:683–692. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- 152.Wright BL, Leiferman KM, Gleich GJ. Eosinophil granule protein localization in eosinophilic endomyocardial disease. N Engl J Med. 2011;365:187–188. doi: 10.1056/NEJMc1103005. [DOI] [PubMed] [Google Scholar]
- 153.Noguchi H, Kephart GM, Colby TV, Gleich GJ. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am J Pathol. 1992;140:521–528. [PMC free article] [PubMed] [Google Scholar]
- 154.Chung HL, Hwang JB, Kwon YD, Park MH, Shin WJ, Park JB. Deposition of eosinophil-granule major basic protein and expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the mucosa of the small intestine in infants with cow's milk-sensitive enteropathy. J Allergy Clin Immunol. 1999;103:1195–1201. doi: 10.1016/s0091-6749(99)70199-5. [DOI] [PubMed] [Google Scholar]
- 155.Kuwahara T, Kobayashi Y, Yun Y, Kanda A, Asako M, Ueki S, Iwai H. Eosinophilic cholecystitis occurred in a patient with refractory eosinophilic airway inflammation: a case report. Allergy Rhinol (Providence) 2019;10:2152656719869607. doi: 10.1177/2152656719869607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hamada S, Ueki S, Miyabe Y, Tsukino M, Hirai T. Focal eosinophilic myositis with Charcot-Leyden crystal formation. Allergol Int. 2020;69:633–635. doi: 10.1016/j.alit.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 157.Kawamura Y, Ikeda R, Hori T, Sasaki T, Miyabe Y, Fukuchi M, Sakamoto K, Ohta N, Kawase T, Katori Y, Ueki S. Sialodochitis fibrinosa: Salivary duct obstruction by eosinophil extracellular traps? Oral Dis. 2020;26:1459–1463. doi: 10.1111/odi.13434. [DOI] [PubMed] [Google Scholar]
- 158.Nogawa H, Suzuki H, Kawabata Y, Ota T, Yuki Y, Katagiri Y, Hino T, Yanagawa N, Ueki S. An unusual case of eosinophilic lung disease with multiple cyst formation. Respir Med Case Rep. 2020;31:101300. doi: 10.1016/j.rmcr.2020.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takeda M, Ueki S, Yamamoto Y, Nara M, Fukuchi M, Nakayama K, Omori Y, Takahashi N, Hirokawa M. Hypereosinophilic syndrome with abundant Charcot-Leyden crystals in spleen and lymph nodes. Asia Pac Allergy. 2020;10:e24. doi: 10.5415/apallergy.2020.10.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fukuchi M, Miyabe Y, Furutani C, Saga T, Moritoki Y, Yamada T, Weller PF, Ueki S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol Int. 2021;70:19–29. doi: 10.1016/j.alit.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walters MJ, Paul-Clark MJ, McMaster SK, Ito K, Adcock IM, Mitchell JA. Cigarette smoke activates human monocytes by an oxidant-AP-1 signaling pathway: implications for steroid resistance. Mol Pharmacol. 2005;68:1343–1353. doi: 10.1124/mol.105.012591. [DOI] [PubMed] [Google Scholar]
- 162.Butterworth AE, Vadas MA, Wassom DL, Dessein A, Hogan M, Sherry B, Gleich GJ, David JR. Interactions between human eosinophils and schistosomula of schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J Exp Med. 1979;150:1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 164.Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Donor sensitivity to basophil activation by eosinophil granule major basic protein. Int Arch Allergy Appl Immunol. 1985;77:216–217. doi: 10.1159/000233791. [DOI] [PubMed] [Google Scholar]
- 165.Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, Schmid I, Radonjic-Hoesli S, Boutet S, Williams GJ, Messerschmidt M, Seibert MM, Cascio D, Zatsepin NA, Burghammer M, Riekel C, Colletier JP, Riek R, Eisenberg DS, Simon HU. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell. 2015;57:1011–1021. doi: 10.1016/j.molcel.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Slifman NR, Loegering DA, McKean DJ, Gleich GJ. Ribonuclease activity associated with human eosinophil-derived neurotoxin and eosinophil cationic protein. J Immunol. 1986;137:2913–2917. [PubMed] [Google Scholar]
- 167.Fredens K, Dahl R, Venge P. The Gordon phenomenon induced by the eosinophil cationic protein and eosinophil protein X. J Allergy Clin Immunol. 1982;70:361–366. doi: 10.1016/0091-6749(82)90025-2. [DOI] [PubMed] [Google Scholar]
- 168.Rosenberg HF, Dyer KD. Eosinophil cationic protein and eosinophil-derived neurotoxin. Evolution of novel function in a primate ribonuclease gene family. J Biol Chem. 1995;270:30234. [PubMed] [Google Scholar]
- 169.Kubach J, Lutter P, Bopp T, Stoll S, Becker C, Huter E, Richter C, Weingarten P, Warger T, Knop J, Müllner S, Wijdenes J, Schild H, Schmitt E, Jonuleit H. Human CD4+CD25+ regulatory T cells: proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood. 2007;110:1550–1558. doi: 10.1182/blood-2007-01-069229. [DOI] [PubMed] [Google Scholar]
- 170.Mishra B, von der Ohe M, Schulze C, Bian S, Makhina T, Loers G, Kleene R, Schachner M. Functional role of the interaction between polysialic acid and extracellular histone H1. J Neurosci. 2010;30:12400–12413. doi: 10.1523/JNEUROSCI.6407-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brix K, Summa W, Lottspeich F, Herzog V. Extracellularly occurring histone H1 mediates the binding of thyroglobulin to the cell surface of mouse macrophages. J Clin Invest. 1998;102:283–293. doi: 10.1172/JCI1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C, Hohenstein B, Hugo C, Uhl B, Reichel CA, Krombach F, Monestier M, Liapis H, Moreth K, Schaefer L, Anders HJ. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol. 2012;23:1375–1388. doi: 10.1681/ASN.2011111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]