Abstract
Bacillus subtilis is a commonly used commercial specie with broad applications in the fields of bioengineering and biotechnology. B. subtilis is capable of producing both biofilms and spores. Biofilms are matrix-encased multicellular communities that comprise various components including exopolysaccharides, proteins, extracellular DNA, and poly-γ-glutamic acid. These biofilms resist environmental conditions such as oxidative stress and hence have applications in bioremediation technologies. Furthermore, biofilms and spores can be engineered through biotechnological techniques for environmentally-friendly and safe production of bio-products such as enzymes. The ability to withstand with harsh conditions and producing spores makes Bacillus a suitable candidate for surface display technology. In recent years, the spores of such specie are widely used as it is generally regarded as safe to use. Advances in synthetic biology have enabled the reprogramming of biofilms to improve their functions and enhance the production of value-added products. Globally, there is increased interest in the production of engineered biosensors, biocatalysts, and biomaterials. The elastic modulus and gel properties of B. subtilis biofilms have been utilized to develop living materials. This review outlines the formation of B. subtilis biofilms and spores. Biotechnological engineering processes and their increasing application in bioremediation and biocatalysis, as well as the future directions of B. subtilis biofilm engineering, are discussed. Furthermore, the ability of B. subtilis biofilms and spores to fabricate functional living materials with self-regenerating, self-regulating and environmentally responsive characteristics has been summarized. This review aims to resume advances in biological engineering of B. subtilis biofilms and spores and their applications.
Keywords: Bacillus subtilis, Biofilms, Spores, Biocatalysis, Bioremediation, Biomaterials, Synthetic biology
Abbreviations: β-Galactosidase, (β-Gal); d-psicose 3-epimerase, (DPEase); Extracellular Polymeric Substance/ Exopolysaccharide, (EPS); Extracellular DNA, (eDNA); Gold nanoparticles, (AuNPs); Green fluorescent protein, (GFP); Isopropylthio-β-d-galactoside, (IPTG); l-Arabinose Isomerase, (L-AI); Menaquinoe-7, (MK-7); Microbial fuel cell, (MFC); Mono (2-hydroxyethyl) terephthalic acid, (MHET); Nanoparticles, (NPs); N-Acetyl-d-neuraminic Acid, (Neu5Ac); N-acetylglucosamine, (GlcNAc); Nickel nitriloacetic acid, (Ni-NTA); Organophosphorus hydrolase, (OPH); Paraoxon, (PAR); Paranitrophenol, (PNP); p-aminophenol, (PAP); Quantum dots, (QDs)
1. Introduction
In the past, biofilms and spores were assumed to pose detrimental effects on human life [1,2], but with the passage of time, their advantages have been discovered and are considered beneficial for certain human applications [3,4]. The beneficial biofilms play an important role in the remediating hazardous pollutants from the environment [5] and for the production of valuable industrial products [6] and hence studied for environmental biotechnology applications. With the help of synthetic tools and engineering techniques, it is now possible to produce value‐added compounds from biofilms and spores for the health care, food additive, animal feedstock, petrol and chemical industry sectors [[7], [8], [9]]. The most commonly spore-forming microorganism are Bacillus, Clostridium, Anoxybacillus, Geobacillus, and Sporolactobacillus. Among all spore-formers, B. subtilis spores possess DNA with a low G + C content and have positive cell wall structure. Therefore, Bacillus subtilis spores and biofilms are of great interest because these are generally regarded as safe [10].
B. subtilis is a motile, gram-positive, facultative aerobic bacterium that is naturally found in soil and vegetation [11]. B. subtilis can switch between growing and dormant state in response to change in nutrient availability. Under the starvation state, it forms inactive dormant cell type called spores. When conditions are favorable for cell growth it can germinate and start its vegetative cycle [12]. B. subtilis biofilms show remarkable three-dimensional (3D) architectural features and used as a reference organism to explore the molecular mechanism and formation of biofilm [13]. Several other recent studies have demonstrated the industrial applications of B. subtilis [[14], [15], [16]]. It has long been known that B. subtilis can survive extreme conditions by producing spores. More recently, it has been demonstrated that B. subtilis sporulation occurs within biofilms, which further protects the bacteria against deleterious chemicals and disinfecting agents or processes [17].
Developments in biotechnology have enabled the application of genetically engineered biofilms and spores as biocatalysts in biotransformation processes. Synthetic biology tools are widely used to design and control B. subtilis biofilms and spores by manipulating regulatory networks, regulating gene expression and engineering cell to cell interactions [18]. Members belonging to the B. subtilis specie are known to yield a broad range of bioactive secondary metabolites, including biosurfactants with antimicrobial properties, such as surfactants, iturins, and fengycins [19,20]. The biosurfactants display better performance in biotechnology applications and have less impact on the environment than conventional surfactants, due to their higher biodegradability and lower toxicity [21]. In addition, the surface display technology has demonstrated that bioactive molecules are present on the spore surface [22]. These recombinant spores have been used for enzyme immobilization that can occur irrespective of the cytoplasmic membrane permeability barrier [23]. Toxic pollutants from wastewater and the surrounding environment can be degraded using bioreactors containing B. subtilis biofilms or combination of multispecies biofilms.
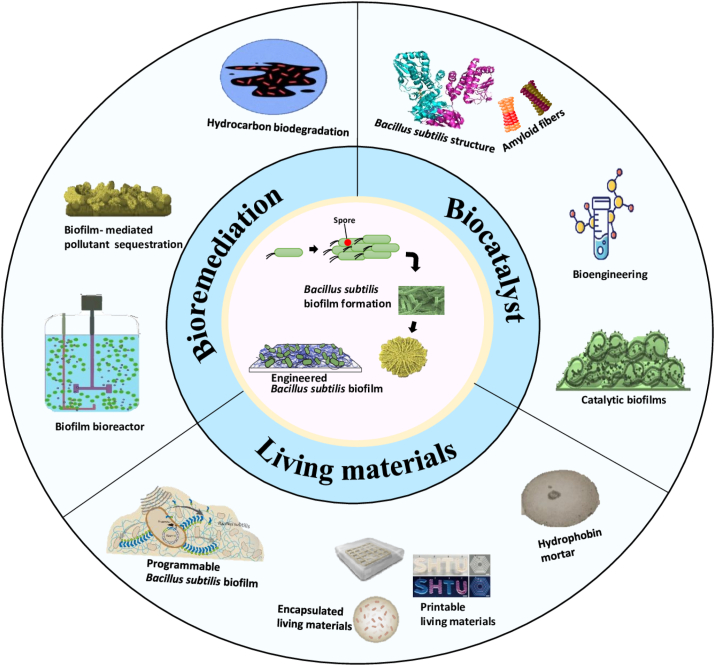
In recent years, the use of B. subtilis biofilms in the field of biotechnology and advanced biomaterials has grown considerably. By programming cellular behavior of B. subtilis biofilms through quorum sensing (QS) signaling molecules in order to understand and control biofilm formation, thus enable broad applications of engineered controllable biofilms for biocatalysis, bioremediation, and biomaterials production [24,25]. For example, the 3D printing of programmable biofilms has been recently developed to synthesize living materials [26]. Based on these recent engineering advancements, previous studies on B. subtilis have provided guidelines for future bioengineering applications. This review focuses on the formation of B. subtilis biofilms and spores and their applications in bioremediation, biocatalysis, and living materials (Fig. 1). The intent is to provide better insight into the synthesis of engineered biofilms, which encompasses the molecular mechanisms involved in formation and regulation of B. subtilis biofilms, for promising applications in bioremediation, biocatalysis, and biomaterials.
Fig. 1.
Graphical summary of applications of B. subtilis biofilms.
2. Formation and regulation of B. subtilis biofilms and spores
2.1. B. subtilis biofilms formation and regulation
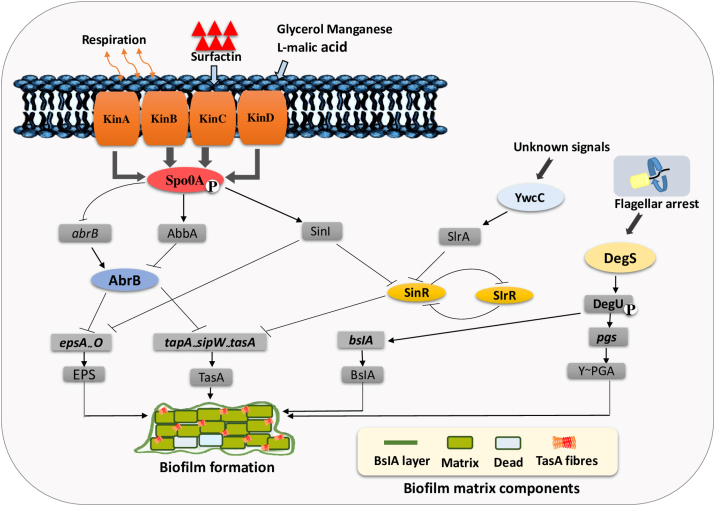
Bacteria can adapt to changing environments using various strategies. These include modulating gene expression to regulate cellular activities like biofilm formation and sporulation [27]. During the past few decades, the non-pathogenic bacterium B. subtilis has emerged as a vital model to study the molecular mechanism, structure and formation of biofilm [13,28]. Various biochemical and cytological studies have demonstrated that B. subtilis biofilms are usually embedded in a self-producing matrix structurally composed of exopolysaccharide (EPS) [[29], [30], [31]]. In the initial stages of biofilm development, interactions among extracellular DNA (eDNA) and EPS are important in biofilm formation, DNA repairing and horizontal gene transfer [32]. In B. subtilis SBE1, the assembly of the complex 3D biofilm architecture is mediated by the interaction among eDNA and EPS [33]. In combination with EPS encoded by epsA-O operon [34], B. subtilis biofilms consist of the TasA protein, the major protein component of TasA fibers [35,36]. A minor protein component TapA is also secreted and participates in vivo in the formation of fibers of TasA [37]. This minor protein is required only for the assembly of TasA fibers in order to promote the stability of TasA and fiber formation in vivo. While, TapA is not necessary for TasA fiber assembly in vitro [38]. Both TapA and TasA proteins are operated and secreted by a signal peptidase SipW encoded by tapA-sipW-tasA operon [39]. In addition, the extracellular matrix is assembled with the minor aid of the BslA protein, which is responsible for forming a hydrophobic coating over the biofilm. Thus, this hydrophobic coating produces a hydrophobic biofilm that floats at the air–liquid interface [40]. Similar to the biofilm EPS matrix component, poly-gamma-glutamic acid (γ-PGA) is an excreted polymeric material found in many environmental isolates of B. subtilis that is important in increasing the robustness of biofilms [41]. Generally, the formation of B. subtilis biofilms involves four sub-networks. As shown in (Fig. 2), Spo0A is the most important transcriptional factor. It regulates the expression of genes in the signal transduction network that is involved in the production of B. subtilis biofilms [42]. The action of specific environmental signals (such as temperature, high iron, oxygen concentration, hydrodynamic effects and food matrix composition) trigger the activation of sensor kinases (KinA-D). KinB is triggered in response to impaired electron transport due to certain environmental stresses (such as low oxygen and high iron) through redox switch. KinA directly binds to NAD+ and sense NAD+/NADH concentrations across the cytoplasmic membrane contributing to the impaired respiration behavior in the cytoplasm [43]. KinC induces biofilm formation in B. subtilis when sensing the leakage of potassium cations from pores in the cytoplasmic membrane generated by surfactin [44,45]. KinD senses organic products such as l-malic acid, glycerol and manganese in plant roots, significantly stimulates biofilm-associated sporulation [46,47]. A multiple constitute of four sensor kinases (KinA, KinB, KinC, and KinD) acts directly or indirectly on Spo0A and promotes the phosphorylation of Spo0A [48]. The phosphorelay following the initial Spo0A phosphorylation by the complex promotes biofilm formation. Therefore, the expression of Spo0A is upregulated as the biofilm matures, which leads to sporulation in B. subtilis biofilms [49].
Fig. 2.
The regulatory network of B. subtilis biofilm formation. Several subnetworks are intertwined to trigger (arrows) or repress (T-bars) the expression of matrix genes involved in biofilm formation. A complex of four kinases (KinA, KinB, KinC, KinD) are activated in response to certain environmental stresses (such as temperature, high iron, oxygen concentration, hydrodynamic effects and food matrix composition). KinA and KinB are activated in response to impaired respiration in cytoplasm, KinC is activated via surfactin production and KinD is activated by glycerol, manganese and l-malic acid production. These sensor kinases initiate the activation of Spo0A through phosphorylation mediating the regulatory pathway for the expression of several matrix genes. Spo0Ã P govern an antirepressor SinI under SinR that derepresses the expression of matrix genes. In addition, SinR represses the regulatory gene slrR. SlrR binds to SinR and forms a heterodimeric complex, which leads to the transcription of the epsA-O and tapA-SipW-tasA operons, resulting in biofilm formation. Similar to SinI, the slrA gene is a paralogous antirepressor for SinR and is repressed by YwcC. Another subnetwork DegU on phosphorylation trigger the transcription of bslA indirectly, which encodes the BslA protein that construct hydrophobic coat over the biofilm. DegU trigger the activation of pgs operon directly that encodes enzymes that catalyze the synthesis of γ-PGA, which enhances biofilm robustness, for more detail see Ref. [13].
To promote the transcription of operons that are necessary for the assemblage of matrix and biofilm parallel channels of anti-repression are activated. The activity of SinR, which is a master transcriptional repressor that regulates biofilm formation in B. subtilis, is regulated by phosphorylated Spo0A (Spo0A-P). SinR is inhibited by SinI, which is transcribed by a small subpopulation of B. subtilis. When the level of the SlrR transcription factor is elevated, SlrR binds to SinR and forms a heterodimeric complex. This complex leads to the transcription of the epsA-O and tapA-SipW-tasA operons [50,51]. Moreover, slrR gene is repressed by SinR, similarly the expression of SinR is inhibited by SlrR protein, resulting in a self-reinforcing double-negative feedback circuit that leads to the de-respression of slrR gene because the repression of SinR is prevented by SlrR. The SlrR-SinR switch can exist in two states. If the expression of SlrR reaches a sufficient level, then it successively remains elevated for several generations. The matrix genes in these circumstances are de-repressed because of low expression levels of SinR (corresponding to chains of matrix producing cells). In other case, the matrix operons are switched off when the expression of SinR is not prevented in case of low levels of SlrR (corresponding to single motile cells). The expression of SlrR is mediated by a transcriptional regulator known as YwcC. Similar to SinI, SlrA represses the activity of SinR and activates the expression of genes in biofilm. AbrB is a transcriptional regulator that attaches to DNA and represses the transcription of matrix genes involved in formation of biofilm in B. subtilis [52]. Spo0A govern the expression of AbrB by two specific ways, (i) the transcription of AbrB is repressed directly by phosphorylated Spo0A, (ii) by triggering the activity of abbA, which encodes AbrB. Furthermore, the expression of both regulatory protein SlrR and biofilm coat protein BslA is repressed by AbrB. The involvement of DegQ protein in biofilm formation has been described as it transfers phosphate group from DegS to DegU [53]. motB gene comprise the stator of flagellum and is required for flagellar rotation. Deletion of motB inhibit flagellar rotation in a cell increases the level of DegU ~ P as well as trigger the transcription of degU results in the biosynthesis of poly-γ-dl-glutamic acid [54]. The expression of pgs operon and bslA is mediated by DegS-DegU signaling pathway, which regulate the complex biofilm formation in B. subtilis. Phosphorylated DegU (DegU-P) indirectly promotes the transcription of target genes, including bslA. This gene encodes the BslA hydrophobic biofilm coat protein in B. subtilis biofilm [55]. Also, the pgs operon that encodes enzymes involved in the activity of biosynthetic genes in the production of γ-PGA is activated directly by DegU. Several studies performed in different biofilm settings using different media conditions have proposed that γ-PGA can influence robustness in biofilm [41,56,57]. The complete regulatory network for the formation of biofilm in B. subtilis is presented in (Fig. 2).
2.2. B. subtilis spores formation (sporulation)
Bacteria can cope with environmental changes through many strategies. One is the sudden change in gene expression that alters cells phenotypically and drives sporulation [27]. During nutrient deprivation, some cells in the biofilm ultimately sporulate [12]. The formation of B. subtilis spores are challenging because their formation is tightly controlled by various regulatory and structural genes. However, sporulation is not a one-step process, but occurs in sub-populations. Probably, sporulation is a bet-hedging strategy that confirms the need to sporulate instead of engaging directly in this energy-intensive process. The first and foremost step in this strategy is the “cannibalistic” behavior that is able to recognize starvation conditions. Cannibalism has secreted killing factors known as Skf and Sdp. During this behavior, the cells kill other cells that are not sufficiently beneficial in biofilm formation, which helps to delay sporulation [58].
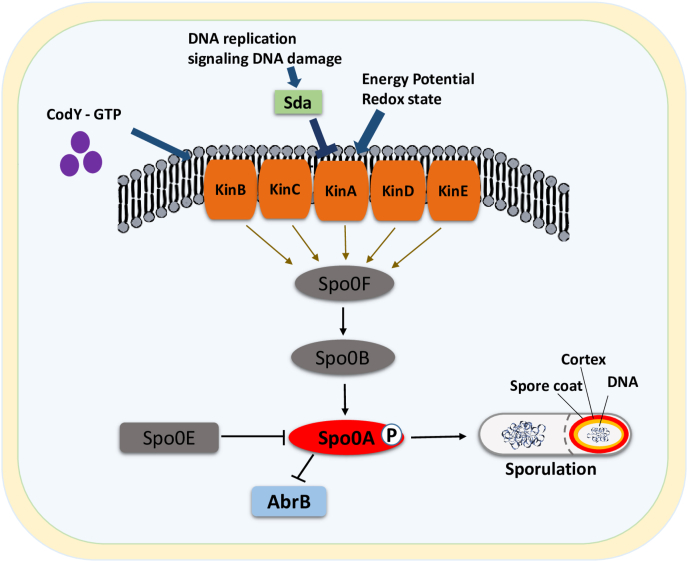
The transcriptional master regulator (Spo0A) involves in the synthesis of biofilm. Spo0A is also responsible for the conversion of B. subtilis states from vegetative to sporulation [59]. The activity of Spo0A is due to histidine sensor kinases (A, B, C, D, and E). KinA is either activated by the inhibition of Sda or the activation of one of its PAS domains which senses energy potential promotes sporulation accordingly. Transcription of kinB is repressed by CodY in the presence of GTP [60]. The activated form of Spo0A has the potential to control the transcription of 121 genes directly, while indirectly exerting control over genes involved in the asymmetric division of cells and over genes that have a role in mediation of specific sigma factors like σE and σF. The overexpression of KinA or KinB is sufficient to trigger the entry into sporulation. In contrast, KinC has a weaker effect on mutation than KinA and KinB [4,61]. A positive feedback mechanism mediates the activation of Spo0A and Spo0A further activated those genes take part in phosphorelay (spo0F and spo0B). Through the phosphorelay, Spo0A activates transcription of some genes while represses transcription of others. One of the genes that is repressed is abrB. The decreasing level of the AbrB protein, a repressor of sporulation genes, initiates the transcription of genes involve in sporulation. The initiation of sporulation is depicted in (Fig. 3). Spo0Ã P also activates transcription of the spoIIA operon encoding sigma F, the spoIIG operon encoding sigma E, and the spoIIE gene required for activation of sigma F in the forespore [62]. In addition, Spo0E consist of a system that specifically dephosphorylates the Spo0A-P and negatively regulates the sporulation initiation pathway to control the proper timing of sporulation [63]. Sda, a small checkpoint protein controls over the activity of Spo0A and preventing sporulation in response to DNA damage by blocking the phospho-transfer from the KinA to Spo0F, hence delays Spo0A activation [64,65]. The levels of Spo0ÃP are responsible for determining the bacterium's developmental choices. Biofilm formation is triggered by low levels of Spo0ÃP while higher levels of Spo0ÃP promote sporulation [66].
Fig. 3.
Initiation of sporulation. During the limited nutrient availability, the sensor kinases (KinA-KinE) initate Spo0F through prohpshorylation. The PAS domain of KinA initiate sporulation which are thought to activate by sensing energy potential or redox state and the repression of kinB transcription by the activity of CodY repressor as it binds to GTP. Phosphorylated Spo0F pass on its phosphate group to Spo0B and subsequently shift the phosphate group to Spo0A, triggering transcriptional regulatory mechanism that leads to sporulation. During DNA damage or impaired DNA replication, sda is activated to prevent sporulation process. Spo0E can dephosphorylate Spo0A. Spo0Ã P represses transcription of abrB gene to initiate transcription of sporulation genes, for more detail of this process refer to Ref. [60].
Besides that, the media and environment conditions both deeply affect spore yield and spore properties. Spore germination is usually triggered by nutrients, non-nutrients and physical treatments. Maximum sporulation usually occurs at an optimal temperature of 37 °C with pH = 8 and high water activity. Similarly, it will delay when it strays from optimum conditions [67]. In evolutionary term, B. subtilis sporulation could be increased by using Dual substrate metabolism. Cells generally use glucose, but they can either use pentoses released from the degradation of plant cell walls. Hence, when glucose is insufficient, cells use pentoses present in the environment before leading to sporogenesis. In short, by using dual substrate metabolism, they can complete their cycle and can enhance their survival. Moreover, cornflour and wheat bran were also considered the best carbon sources for increasing sporulation [68].
In addition, sporulation can also be increased by using nitrogen sources like corn steep liquor, soybean flour, and yeast extract. The optimized condition of these sources produced spores as high as 1.52 ± 0.06 × 1010 spores/mL under flask cultivation conditions. Moreover, during scale-up, 1.56 ± 0.07 × 1010 spores/mL were produced in 30 L fermenter after 40 h of cultivation [68]. In another study, chemical medium was used to enhance sporulation. The medium was optimized to achieve the maximum production of spores. Hence, the obtained calculated amount of spores was 3.6 × 1010 spores/mL [69]. In the latest research, Two-stage solid-state fermentation has been optimized to enhance B. subtilis growth and sporulation. As a result, the effective cell number of B. subtilis reached 1.79 × 1010/g dry medium after fermentation for 72 h, which was 29.7 % and 8.48 % higher than that of conventional fermentation for 72 h and 48 h, respectively. Hence, the optimal two-stage fermentation could significantly increase the cell number of B. subtilis efficiently [70].
3. Engineered B. subtilis biofilms for Bioremediation
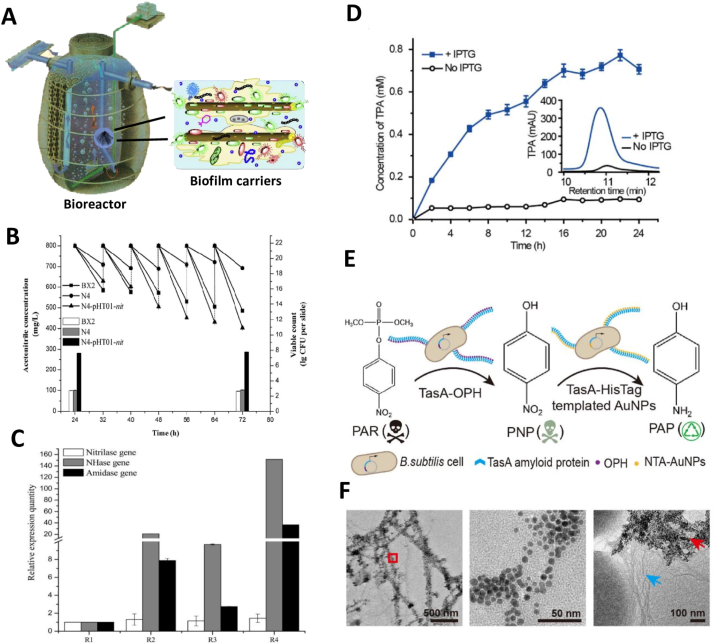
Biofilms act as a tool in bioremediation that can allow new technologies to remain environmentally sustainable, if methods are correctly developed and applied. Biofilm-mediated bioremediation is cost-effective, as the end product is nonpolluting, which might also contribute to a low environmental footprint [71]. Technologies utilizing the potential role of biofilms as a carrier for municipal and industrial wastewater treatment are environment friendly and are of great interest. Biofilm based design employing biofilm carriers are widely used for the bioremediation of pollutants from wastewater. Bio-carriers with strong biofilm adhesion act as redox mediators to accelerate the biodegradation of contaminants from wastewater. Zhang et al. [72] developed an environment-friendly inorganic modified basalt fiber (MBF) for more efficient wastewater treatment. The MBF showed strong B. subtilis adhesion and shorter biofilm formation time providing an alternative to conventional bio-carriers for the biotransformation of pollutants in industrial effluents (Fig. 4A) [72].
Fig. 4.
Biofilm-mediated engineering in B. subtilis for bioremediation process. (A) B. subtilis adhesion and biofilm formation on modified basalt fibers (MBF) bio-carrier for wastewater treatment [72]. (B) Acetonitrile degrading capability of B. subtilis N4-pHT01-nit after waste water treatment [79]. (C) Cell growth of B. subtilis N4/pHTnha-ami and organonitriles (acetonitrile, acrylonitrile, crotononitrile) degradation by NHase, amidase and nitrilase genes [80]. (D) High-performance liquid chromatography analysis of (MHET) degradation using a ΔtasAΔsinRΔeps/TasAMHETase biofilm [26]. (E) Two-step biocatalytic cascade reaction using biofilms derived from co-cultured strains of TasA-HisTag and TasA-OPH for biodegradation of organophosphate pesticides. TasA-OPH biofilm comprising functional (OPH) enzyme that degrades (PAR) into (PNP). Next, Immobilization of gold nanoparticles (AuNPs) on the TasA-HisTag biofilm further degrades PNP to (PAP) [26]. (F) Transmission electron microscopy images showing nickel nitriloacetic acid (Ni-NTA)-decorated AuNPs embedded in biofilms containing TasA-HisTag nanofibers (left and middle) and complex biofilms containing TasA-OPH (blue arrow) and TasA-HisTag nanofibers embedded with AuNPs (red arrow; right). The middle image is a higher magnification view of the area marked with a red square in the image to the left [26].
The potential of B. subtilis biofilms for bioremediation processes has been recently demonstrated. The effective biodegradation of harmful volatile organic compounds such as benzene, toluene and xylene (BTX) is more efficient with co-culture of B. subtilis specie with other species than individual specie. A consortium of B. subtilis strain DM-04 and M and NM strains of P. aeruginosa are allowed to spread and thrive on numerous substrates of hydrocarbon. In addition, the coalition use polyaromatic hydrocarbons as a carbon source [73]. Recently, isolated Biofilm forming B. subtilis strain DKT from soil has been shown to degrade 1,2-dichlorobenzene as it utilizes benzene and chlorobenzene as a carbon source [74].
Previous studies documented the pronounced capacity of B. subtilis biofilms to immobilize trivalent chromium Cr(III) [75,76]. Bioremediation of environmental pollutant Cr(III) through B. subtilis biofilms in tannery industry have been well documented. Biofilms generated from a mixture of B. subtilis and B. cereus produced greater surface area on rough sand and were capable of reducing 98 % of Cr(III) [77]. The development of integrated methods can aid in the application of biofilms for the bioremediation of potentially harmful compounds. Artificially developed biofilms can be used for the bioremediation of toxic pollutants in the environment [78].
Genetic engineering in biofilm forming bacteria boosts its performance in order to completely degrade toxic pollutants and halt their exposure into the environment. A genetically engineered biofilm-forming bacterium B. subtilis N4-pHT01-nit can be used to degrade acetonitrile from wastewater. This genetically engineered strain was constructed by cloning a novel nitrilase (nit) gene from bacterium, Rhodococcus rhodochrous BX2 that degrade toxic compound nitrile into a biofilm-forming bacterium B. subtilis N4, displayed recombinant protein upon IPTG induction (Fig. 4B) [79]. Further, Li et al. [80] cloned Nitrile hydratase (nha) and amidase (ami) genes into the B. subtilis N4 which shows a strong biofilm forming ability and construct a genetically modified B. subtilis N4/pHTnha-ami, which completely degrade organonitriles from the wastewater. Furthermore, to promote biofilm formation and bacterial adherence modified polyethylene carriers with positive charge was also applied moving bed biofilm reactor (MBBR) (Fig. 4C) [80].
Huang et al. [26] constructed B. subtilis biofilm that produced TasA-MHETase nanofibers on its surface. Using this engineered biofilm, a harmful organic compound mono (2-hydroxyethyl) terephthalic acid that is produced on massive scale industrially degraded into the less harmful terephthalic acid (Fig. 4D). Furthermore, the biodegradation of organophosphate pesticides has been demonstrated using a two-step biocatalytic cascade reaction mediated by biofilms derived from co-cultured strains of TasA-organophosphorus hydrolase (OPH) and TasA-HisTag. TasA-OPH biofilm comprising functional OPH has reportedly degraded the paraoxon (PAR) pesticide into a less toxic paranitrophenol (PNP) (Fig. 4E). Further degradation of PNP into non-toxic p-aminophenol (PAP) is achieved by immobilization of gold nanoparticles (AuNPs) on TasA-HisTag biofilms (Fig. 4F) [26].
4. Engineered B. subtilis biofilms and spores for Biocatalysis
4.1. Engineered B. subtilis biofilms for biocatalysis
The applications of biocatalysts in the biotransformation process in bio-related industries have been increasing recently [81]. The sensitivity of enzymes to extreme pH, temperature, and mechanical stress has limited their applications for biotransformation [82]. This limitation can be addressed using biofilms, which are robust structures protected by a polysaccharide matrix that confer resistance to extreme physical and chemical stresses [83]. Bacterial biofilms are effective biocatalysts for many biomaterials production, carrying enzymes, amino acids, antibiotics, chemicals, butanol, bioethanol, polysaccharides, and surfactants. Moreover, electricity has been produced by using bacteria that catalyzes biochemical reactions in a microbial fuel cell (MFC) biofilm-based system [84]. Biofilms either derived from single, multiple or from a natural community are widely used for the commercial production of acetic acid, butanol, ethanol, lactic acid, propionic acid, succinic acid, styrene oxide and electricity [85]. Recently, biofilm reactors can be considered as promising tools which are used to enhance the production of value-added products in research laboratories. In addition to enhanced tolerance to toxic substances and products, biocatalysts developed based on biofilms exhibit long-term activity and have continuous processing capabilities [86,87].
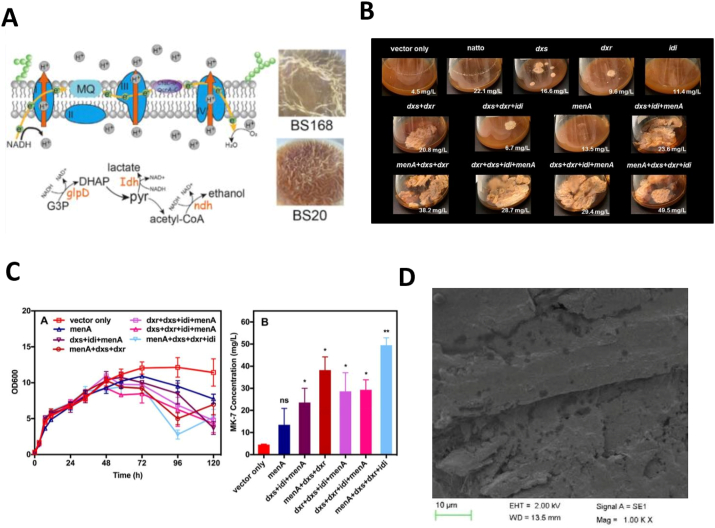
An active member of vitamin K family, Menaquinoe-7 (MK-7) is produced in a batch fermentation system comprising B. subtilis natto was demonstrated to follow a mixed-metabolite pattern [88]. The biofilm formation in B. subtilis affect the synthesis of MK-7 as it facilitates the production [89,90], although the mechanism of biofilm in the synthesis of MK-7 has not been revealed yet. Therefore, several kind of engineering strategies have been exposed to biofilm forming B. subtilis to understand the essential role of biofilm in MK-7 synthesis. Overexpression of menaquinol-cytochrome c reductase (QcrA-C) in B. sutbilis BS20 promote a strong biofilm formation regulating the components of cell membrane and electron transfer by delivering more electrons results in an increase MK-7 synthesis (Fig. 5A). Overexpression of oxalate-decarboxylase (OxdC) also results in an increase production of MK-7 [91]. Ma et al. [92] constructed 12 different strains of B. subtilis 168 by overexpressing different combinations of enzymes Dxs, Dxr, Idi, and MenA. The construct menA-dxs-dxr-idi showed a large amount of biofilm formation results in an increased production of MK-7 to 50 mg/L, a 1.7 fold-increase compared to the other 11 gene cluster (Fig. 5B). These Results showed the increases MK-7 Production Induces biofilm formation confirm the relationship between MK-7 synthesis and biofilm formation in B. subtilis (Fig. 5C) [92].
Fig. 5.
Biocatalyst performance of B. subtilis biofilm. (A) Electron transfer chain role and biofilm formation in B. subtilis 168 and BS20 in MK-7 synthesis [91]. (B) Pellicle biofilm formation and MK-7 production in shake-flask cultures by identical strains [92]. (C) Growth curve and MK-7 production by strains co-overexpressing the MEP pathway and downstream menA gene [92]. (D) SEM showed the CFT carrier was completely covered with biofilm for bioconversion of vanillin after 40 h of operation [93].
Additionally, the biocatalytic system has been used for the conversion of ferulic acid to vanillin, a valuable compound widely used as a flavour in the food, beverage, perfumes and medical industries. Under the optimized conditions at 35 °C, 9.0 pH and 200 rpm for 20 h, vanillin bioconversion in stirring bioreactor packed with carbon fiber textiles (CFT) carrier biofilm formed by immobilized B. subtilis cells showed the highest production rate of 1.84, which was 3.61 folds higher than those obtained in a free cell system. Results showed that vanillin bioconversion under these optimized conditions is closely related to the cellular activity and growth. Holes and channels in CTF carrier showed ESP production and biofilm formation by B. subtilis (Fig. 5D) [93]. Further, Box-Behnken design (BBD) approach of response surface methodology (RSM) was used to evaluate the bioconversion of ferulic acid to vanillin after slightly modifying the optimal conditions in stirring bioreactor packed with a carbon fiber textiles (CFT) carrier biofilm formed by B. subtilis. The tests revealed that vanillin molar yield (M) and ferulic acid conversion efficiency (E) were 57.42 % and 93.53 %, respectively, considering this biocatalytic system a successful approach for the production of vanillin [94].
4.2. Engineered B. subtilis spores as surface display for biocatalysis
The spore based bio-catalysis platform has potential to produce and self-assembling of multimeric enzymes on the surface of microbial spores. Such enzymes can be achieved by spore surface display technology. The Bacillus spore surface display (BSSD) technique is one of the unique tools in the field of molecular biology that localizes the foreign protein on spore surface through fusion vectors using two genes encoding anchor protein and target protein. Such expressed proteins also show greater stability and resistance as like spores. Moreover these
Proteins can easily be purified with high recovery rate [95].
Gram positive bacteria are attractive vehicles for surface display as they have simple and rigid cell wall. Among them B. subtilis is more favorable choice because it is well known probiotic and its spores has high stability and resistance against harsh conditions. Hence literature scanning indicates that B. subtilis spores are of great interest for displaying enzymes as a biocatalyst. Enzymes as a biocatalyst have remarkable advantages in industrial area like pharmaceuticals, agro-chemicals and food ingredients. Mostly, enzymes are capable of catalyzing reactions in aqueous solution instead of organic solvent so best alternative for conventional chemical processes. The demand for industrial enzymes has been increasing hence surface display technology by using B. subtilis spores is most effective technique for immobilization of enzyme and to meet the industrial demand for preparation and stabilization of biocatalyst [96].
Biocatalysts are extensively used for the biosynthesis of large and complex compounds. Biocatalysts are also used for the production of fine and bulk chemicals through chemosynthetic processes and for applications in the pharmaceutical industry. Table 1 provides applications of spores and biofilms as biocatalysts that use spore coat protein as an anchoring motif and provide applications respective to their products.
Table 1.
Applications of spore and biofilm based biocatalysts.
| Biocatalyst |
Strategy |
Production |
Applications |
Ref. |
|
|---|---|---|---|---|---|
| Spore based biocatalysts | |||||
| Spore surface display |
|||||
| Spore coat protein | Target genes | ||||
| N-acetyl-d-neuraminic acid Aldolase | CotG | nanA | 4.9 g/L/h N-acetyl-d-neuraminic acid | Pharmaceutical | [97] |
| β-galactosidase | CotX | bgaB | 8.8 g/L lactulose from 200 g/L lactose and 100 g/L of fructose | Pharmaceutical and dairy industry | [98] |
| CotG | araA | 4.3 g/L/h d-tagatose | Food industry | [99] | |
| CotG | lacZ | 8.1 g/L octyl-d-galactopyranoside | Chemical industry | [23,95] | |
| d-psicose 3-epimerase | CotG | Dpe | 85 g/L d-allulose from 500 g/L d-fructose | Pharmaceutical and food industry | [100] |
| Haloalkane Dehydrgenase | CotG | dhaA | 1.74 ± 0.06 U/mL hydrolyzing activitiy of haloalkane dehalogenase | Bioremediation | [101] |
| Phytase | CotG | appA | The phytase activities are 82.61, 91.62, and 63.08 U/108 spores from pHT304-CotG-AppA, pHT304-CotG-A-AppA, and pHT304-CotG-B-AppA, respectively. | Animal Probiotic | [96,102] |
| Biofilms as biocatalyst | |||||
| Oxalate decarboxylase and menaquinol-cytochrome c reductase | Overexpression of OxdC and QcrA-C | 200–310 mg/L vitamin K in 15 L bioreactor | Therapeutic Role | [91] | |
| 1-deoxy-d-xylose-5-phosphate synthase (dxs), 1-deoxy-d-xylose-5-phosphate reducto-isomerase (dxr), Isopentenyl-diphosphate Delta-isomerase (idi) and 1,4-dihydroxy-2-naphthoate octaprenyltransferase (menA) |
Overexpression of dxs, dxr, idi, and menA | 50 mg/L vitamin K | Therapeutic Role | [92] | |
5. Bacillus subtilis biofilms and spores as living functional materials
5.1. Engineered biofilms as living materials
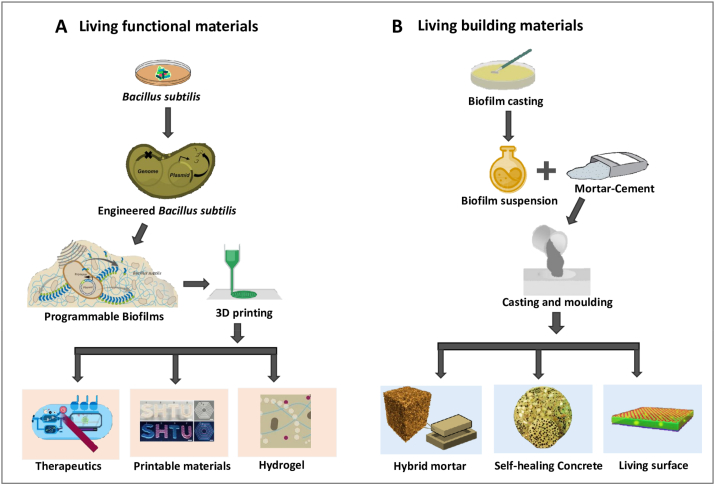
B. subtilis biofilms have been genetically programmed to produce living functional materials that exhibit self-regenerating and self-replicating characteristics and can respond to the environment. B. subtilis TasA amyloid machinery was engineered to produce TasA-R fusion proteins. These biofilms secreted functional domains that congregate into multiple extracellular nano-framework. These living materials with unique functional characteristics have potential applications at the nanoscale level in biotechnology industries. B. subtilis amyloid machinery can synthesize programmable TasA fusion protein with diverse functional domains upon exposure to chemical inducers. The fusion proteins self-organize into fibrous extracellular matrix on cell surfaces. The viscoelastic behavior of living B. subtilis biofilms enable their application in 3D printing. The fabricated living materials when captured in hydrogels retain their natural activity and numerous cellular functions such as self-regeneration (Fig. 6A) [26].
Fig. 6.
Applications of B. subtilis biofilms as biomaterials. (A) Engineering B. subtilis biofilms as living functional materials. Three-dimensional complex printing of biofilms into multiple printable designs, hydrogel and living therapeutics. (B) B. subtilis biofilms as living building materials, Casting and moulding of B. subtilis biofilm with mortar and cement for the construction of bio-bricks such as hybrid mortar, self-healing concrete and living surfaces.
Zhang et al. [103] successfully constructed a strong adhesive living glue using B. subtilis biofilms. The biofilms were genetically engineered by fusing TasA and BslA with mussel foot proteins (MfpThe Mfps) proteins were further modified by tyrosinase. The viscidity of biofilms was enhanced by adding metal ions that interacted with EPS. The produced biofilm-based glue that was reported adjustable and self-generating [103].
B. subtilis biofilm surfaces exhibit wetting resistance towards water. In addition to various organic solvents and biocides, B. subtilis biofilm surfaces also exhibit wetting resistance towards ethanol concentrations of up to 80 %. The wetting resistance properties of B. subtilis biofilms are similar to those of polytetrafluorethylene [104]. Additionally, B. subtilis biofilms exhibit resistance toward the penetration of gaseous vapors. The wetting resistance property of biofilms is attributed to EPS and protein components of the extracellular matrix [105]. The secretion of surfactin, a biomolecule enhances B. subtilis biofilm spreading by lowering the effective surface tension of liquids also enhances the wetting resistance property of B. subtilis biofilms [106]. Due to the strong hydrophobic surface characteristics, these enriched biofilms are added to hybrid mortars can enhance the wetting resistance properties and suppress the absorption of water via capillary forces. Thus, the wetting resistance property of biofilms enables their application in civil engineering. Biofilms are regenerative material and hence used in the manufacturing of living building materials such as hybrid mortar, self-healing concrete and living surfaces (Fig. 6B). The addition of a hybrid mortar with B. subtilis 3610 biofilms on enriched Luria Bertani agar (LB plus agar) with a contact angle of approximately 110° improves the wetting resistance property when compared with the unmodified mortor with a contact angle of approximately 30° [107].
5.2. Bacillus spores as living materials and recent advances in synthetic biology
Living materials are live cells embedded within a structural scaffold make hybrid living materials. These materials can be used for various applications like sensing, chemical or material production, and bioelectronics. A challenge in dealing with such materials is to keep the cells alive for a long period of time under stressful conditions [108]. As spores are resistant to extreme environmental stresses, such as high temperature and pressure, oxidizing agents, and acid or alkaline solutions, they can be used as functional living materials. Additionally, spores can survive for years due to dipicolinic acid and by adopting a wrinkled shape to withstand osmotic stress [109,110]. A 3D printer has been designed to make objects with embedded spores [111]. The construction of 3D printed materials having spores are more advantageous compared with materials containing vegetative cells. These materials enable the vital cell functions to be used for various applications that require long-term storage, in-field functionality, or exposure to uncertain environmental stresses.
5.2.1. Spores as living sensors
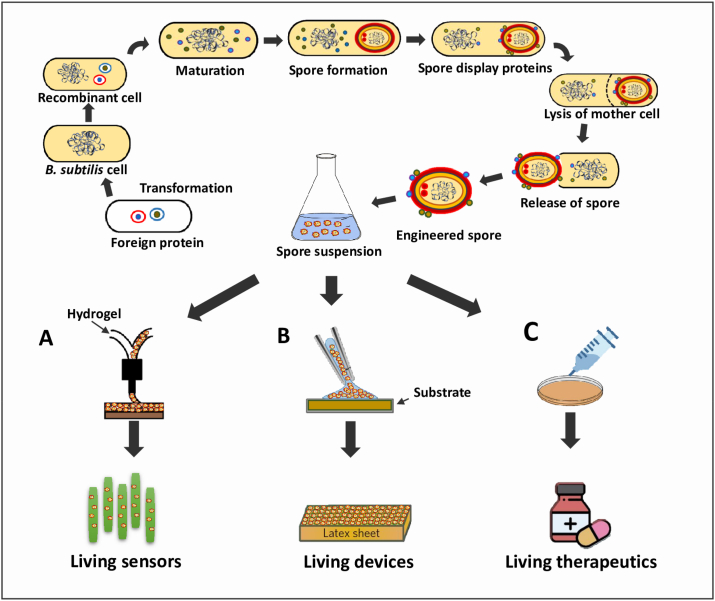
The modified printer, termed the MakerBot Replicator, generates objects by extruding plastic through a high temperature nozzle. The nozzle is redesigned in such a way that can mix two streams to form the bioink before printing. The liquid agarose in 3D printing exhibits shear thinning and can be printed with various thermophilic Bacillus species. The fraction and distribution of viable cells in the printed structure can be increased using the purified spores. The spores remain distributed throughout the material and germinate to perform their genetically engineered functions, such as responding to chemicals such as IPTG, Xylose (Xyl), Vanillic acid (Van) and cuminic acid (Cum). Furthermore, the spores contain dried material and hence can be stored at ambient temperature for a prolonged period. The spores germinate upon rehydration, reconstitute their printed shape, and regain activity. The spores are resistant to various agents that include ethanol, high osmolarity, UV light and germinate quickly (Fig. 7A) [111].
Fig. 7.
Engineering B. subtilis spores as a resilience living functional materials (A) Three-dimensional printing of engineered spores with liquid hydrogel for the productions of living biosensors. (B) A layer of spores on silicon microcantilevers or latex rubber sheets for the construction of living devices. (C) 3D printing of engineered spores with polymer based hydrogel for the production of living therapeutics.
5.2.2. Spores as living devices
The living devices are generally designed to harvest the energy hence provide contributions to attenuate the global energy and environmental crisis. Bacillus spores have been used to build an energy-harvesting device. The authors reported that the cotE gerE mutant spores increased the energy density by approximately 2-fold compared to that of wild-type spores. These mutant spores self-assembled into dense sub-micrometer thick monolayers on substrates, such as silicon microcantilevers and elastomer sheets, to generate bio-hybrid hydromorphic actuators [112]. In a study evaluating the mechanical response of Bacillus spores to water gradients, the energy density of Bacillus spores (>10 MJ/m3) was higher than that of synthetic water-responsive materials (Fig. 7B) [113].
5.2.3. Spores as living therapeutics
Living therapeutics can be used for the delivery of drugs inside the body as well as for the treatment of skin infections caused by pathogens. B. subtilis bacteria also have been programmed to sense and respond to the wound-infecting bacterium Staphylococcus aureus, by emitting green fluorescence. Hence hydrogel patches comprised of B. subtilis spores were printed using the 3D printer that is suitable for human wound model. B. subtilis strains capable of producing antibiotics lysostaphin and thiocillin in 3D printed wound shaped hydrogel patches to kill S. aureus. In short, B. subtillis spores entrapped in soft and hydrated hydrogels can be used as cost effective medical bandages with potentital antimicrobial properties for wound treatment [114].
In addition, B. subtilis spores are also applied to treat fungal infections. For example, B. subtilis spores embedded in a smart and adaptable gel, made of thermo-responsive polymer Pluronic F-127 are used to make responsive against superficial fungal infection. This gel possess the quality of conversion from liquid to hydrogel state when temperature increases to 37 °C (Fig. 7C) [115].
6. Conclusions
As B. subtilis biofilms and spores are programmable and resistant to environmental stress, they can be utilized for a variety of applications. Biofilms and spores are widely used for the production of industrial chemicals and electricity (through MFCs). Additionally, biofilms are important for industrial production of enzymes. The spore outer coat provides a suitable surface for displaying heterologous antigens using coat proteins, such as Cot A, B, C, and G.
B. subtilis biofilms and spores also have potential applications in bioremediation. The genetically engineered B. subtilis biofilms are used for bioremediation of toxic pollutants from wastewater. Additionally, biofilms derived from laboratory-constructed B. subtilis strains can be engineered to produce compounds that can degrade or eliminate industrial waste from the environment. The ability of B. subtilis to degrade pollutants and form biofilms can be used to devise novel strategies for bio-oxidation of toxic pollutants from the environment. Spores are considered more efficacious than vegetative cells for enzymatic metal transformations due to their resilient nature. In addition, spores can be used without the concern of a toxic environment and no added nutrients are needed. Further studies are needed for long-term application of spores. Biofilms enriched with B. subtilis exhibit wetting resistance toward water, organic solvents, and biocides.
The recombinant enzymes produced using spores are stable at high temperature and pressure. Additionally, the enzymes displayed on the spore surface decrease the cost of industrial production as the immobilized enzymes can be recycled and re-purified. Enzymes displayed on the spore surface are also beneficial for the integration of chemical and enzymatic reactions for the chemoenzymatic synthesis of complex compounds. The production of antimicrobial compounds, such as subtilin, from spore-forming B. subtilis biofilms is one of the success stories of the enzyme industry. The production yield can be further enhanced using the new biofilm-integrated systems to remove current production barriers. Moreover, the production of new food ingredients, such as Neu5Ac and d-tagatose, has contributed to the progress in the food industry.
Recent studies have demonstrated the production of synthetic biological materials by 3D printing. Future studies should focus on improving the applications of these 3D printed materials. Recent methodological developments in material science and synthetic biology have enabled the incorporation of many useful functionalities into 3D printed structures. Biofilm-integrated nanofiber displaying is a strategy for the molecular programing of B. subtilis biofilm extracellular matrix to produce extra functional amyloid proteins and diverse domains, which can be a versatile nano-biotechnology platform for developing new materials with programmable functions.
In conclusion, the current developments in engineering B. subtilis biofilms and spores have contributed to the progress of bioremediation, biocatalysis, and biomaterial engineering. Further developments will enable applications in various disciplines, such as synthetic biology and advanced manufacturing. These technologies will improve human-made designs and industrial applications.
7. Future prospects
In this review, the main purpose is to provide information about the attributes of engineered B. subtilis biofilms and spores so that one can take advantage and could broaden the applications by using different genetic tools. In this regard, systematic metabolic engineering can provide direction for the future developments of engineered strains of B. subtilis. As we know, B. subtilis is a commonly used specie because it has a broad array of mature genetic tools, promoters, and plasmid expression systems, which can be used in metabolic engineering, protein expression, and synthetic biology. Furthermore, programmable B. subtilis biofilms have the ability to express proteins and different molecules. Therefore, they can be exploited in different applications like biosensors and biotherapeutics, as these applications also require the secretion of proteins. Thus, B. subtilis biofilms and spores are considered a new form of living functional material that can regenerate and possess other attributes as well. Such biofilms and spores will pave the path for developing many conceivable new classes of complex multifunctional materials, dynamic and regenerative nanotechnologies.
Currently, many researchers are making efforts to construct multiple genetically engineered bacterial biofilm domains as a platform possessing genetic fusions of different functional proteins and biofilm proteins, which may serve as a bioscaffold candidate for biocatalysis. Production of living responsive materials that can sense environmental signals and respond intelligently will pave the way for constructing on-demand biofilm living functional materials. These functional materials can operate as a pollutant sensor and absorbent and could be used in applications such as water, air filtration and metal ion sequestration. Furthermore, B. subtilis biofilms and spores have been known to attract people interest for its remarkably great role in the biotransformation of complex compounds into valuable products. Researchers are keen to develop more and more successful biocatalytic systems for the production of highly value-added products. Moreover, with the advancement of synthetic biology, it would be possible to use biofilm and spores as biotherapeutics as they show resistance to the unsuitable environment. In the future, with the progress of live biotherapeutics, we will be able to control various diseases efficiently. In short, merging interdisciplinary sciences could make spores viable options for the development of multifunctional living materials equipped with diverse functionalities.
Moreover, combining synthetic biology and material sciences with bioengineering applications for the fusion of living and non-living materials could result in the advancement of desirable nanomaterials with completely novel functionalities would be highly complementary to this effort. For example, biodegradable materials that can self-assemble and re-engineer could replace non-biodegradable materials. Bioremediation applications of living responsive materials for the screening and removing toxic pollutants from the industrial effluents will surely boast the industry. Furthermore, living building materials with an ability to self-repair after damage could be a breakthrough in the field of civil engineering. Conclusively, all these objectives could be achieved with the help of synthetic biology in revolutionizing the production of functionalized biopolymers and biomolecules, which could provide a key template for the advancement of engineered living materials.
Conflict of interest
The authors declare no conflict of interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2020YFA0908900), the Natural Science Foundation of Shanghai (19ZR1477100), and the National Natural Science Foundation of China (31872728).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Jiaofang Huang, Email: huangjf@ecust.edu.cn.
Yingping Zhuang, Email: ypzhuang@ecust.edu.cn.
References
- 1.Donlan R.M. biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 2.Logan N. Bacillus and relatives in foodborne illness. J Appl Microbiol. 2012;112:417–419. doi: 10.1111/j.1365-2672.2011.05204.x. [DOI] [PubMed] [Google Scholar]
- 3.Morikawa M. Beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J Biosci Bioeng. 2006;101:1–8. doi: 10.1263/jbb.101.1. [DOI] [PubMed] [Google Scholar]
- 4.Lin P. Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl Microbiol Biotechnol. 2020;104:2319–2331. doi: 10.1007/s00253-020-10348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards S.J., Kjellerup B.V. Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl Microbiol Biotechnol. 2013;97:9909–9921. doi: 10.1007/s00253-013-5216-z. [DOI] [PubMed] [Google Scholar]
- 6.Strieth D., Ulber R., Muffler K. Application of phototrophic biofilms: from fundamentals to processes. Bioproc Biosyst Eng. 2018;41:295. doi: 10.1007/s00449-017-1870-3. 12. [DOI] [PubMed] [Google Scholar]
- 7.Velmourougane K., Prasanna R. Agriculturally important microbial biofilms: present status and future prospects. J Basic Microbiol. 2017;57:548–573. doi: 10.1002/jobm.201700046. [DOI] [PubMed] [Google Scholar]
- 8.Srey S., Jahid I.K., Ha S.-D. Biofilm formation in food industries: a food safety concern. Food Contr. 2013;31:572–585. [Google Scholar]
- 9.Tian X. Characteristics of a biofilm photobioreactor as applied to photo-hydrogen production. Bioresour Technol. 2010;101:977–983. doi: 10.1016/j.biortech.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Setlow P., Johnson E.A. Spores and their significance. Food Microbiol: fundamentals and frontiers. 2019:23–63. [Google Scholar]
- 11.Kovács Á.T. Bacillus subtilis. Trends Microbiol. 2019;27:724–725. doi: 10.1016/j.tim.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Branda S.S. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci U S A. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlamakis H. Sticking together: building a biofilm the Bacillus subtilis way. Nat Rev Microbiol. 2013;11:157–168. doi: 10.1038/nrmicro2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Earl A.M., Losick R., Kolter R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008;16:269–275. doi: 10.1016/j.tim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dijl J.M., Hecker M. Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Factories. 2013;12:3. doi: 10.1186/1475-2859-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahcheraghi S., Ayatollahi J., Lotfi M. Applications of Bacillus subtilis as an important bacterium in medical sciences and human life. Trop J Med Res. 2015;18:1–4. [Google Scholar]
- 17.Wahlen L. Production and analysis of a Bacillus subtilis biofilm comprised of vegetative cells and spores using a modified colony biofilm model. J Microbiol Methods. 2018;148:181–187. doi: 10.1016/j.mimet.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y. Synthetic biology toolbox and chassis development in Bacillus subtilis. Trends Microbiol. 2019;37:548–562. doi: 10.1016/j.tibtech.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Harwood C.R. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev. 2018;42:721–738. doi: 10.1093/femsre/fuy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaspar F., Neubauer P., Gimpel M. Bioactive secondary metabolites from Bacillus subtilis: a comprehensive review. J Nat Prod. 2019;82:2038–2053. doi: 10.1021/acs.jnatprod.9b00110. [DOI] [PubMed] [Google Scholar]
- 21.Gudiña E.J. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao C. Chemoenzymatic synthesis of N-acetyl-D-neuraminic acid from N-acetyl-D-glucosamine by using the spore surface-displayed N-acetyl-D-neuraminic acid aldolase. Appl Environ Microbiol. 2011;77:7080–7083. doi: 10.1128/AEM.05601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon S.J., Jung H.C., Pan J.G. Transgalactosylation in a water-solvent biphasic reaction system with beta-galactosidase displayed on the surfaces of Bacillus subtilis spores. Appl Environ Microbiol. 2007;73:2251–2256. doi: 10.1128/AEM.01489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F. A synthetic quorum sensing system reveals a potential private benefit for public good production in a biofilm. PloS One. 2015;10 doi: 10.1371/journal.pone.0132948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalamara M. Social behaviours by Bacillus subtilis: quorum sensing, kin discrimination and beyond. Mol Microbiol. 2018;110:863–878. doi: 10.1111/mmi.14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J., Liu S., Zhang C. Programmable and printable Bacillus subtilis biofilms as engineered living materials. Nature. 2019;15:34. doi: 10.1038/s41589-018-0169-2. 1. [DOI] [PubMed] [Google Scholar]
- 27.Tasaki S., Nakayama M., Shoji W. Morphologies of Bacillus subtilis communities responding to environmental variation. Dev Growth Differ. 2017;59:369–378. doi: 10.1111/dgd.12383. [DOI] [PubMed] [Google Scholar]
- 28.Cairns L.S., Hobley L., Stanley-Wall N.R. Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms. Mol Microbiol. 2014;93:587–598. doi: 10.1111/mmi.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elsholz A.K.W., Wacker S.A., Losick R. Self-regulation of exopolysaccharide production in Bacillus subtilis by a tyrosine kinase. Genes Dev. 2014;28:1710–1720. doi: 10.1101/gad.246397.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai Y. Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis. mBio. 2012;3 doi: 10.1128/mBio.00184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López D., Vlamakis H., Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zafra O. Extracellular DNA release by undomesticated Bacillus subtilis is regulated by early competence. PloS One. 2012;7 doi: 10.1371/journal.pone.0048716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng N. The exopolysaccharide–eDNA interaction modulates 3D architecture of Bacillus subtilis biofilm. BMC Microbiol. 2020;20:115. doi: 10.1186/s12866-020-01789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu F. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 35.Steinberg N. The extracellular matrix protein TasA is a developmental cue that maintains a motile subpopulation within Bacillus subtilis biofilms. Sci Signal. 2020;13 doi: 10.1126/scisignal.aaw8905. [DOI] [PubMed] [Google Scholar]
- 36.Branda S.S. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 37.Romero D. Functional analysis of the accessory protein TapA in Bacillus subtilis amyloid fiber assembly. J Bacteriol. 2014;196:1505–1513. doi: 10.1128/JB.01363-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Earl C. The majority of the matrix protein TapA is dispensable for Bacillus subtilis colony biofilm architecture. Mol Microbiol. 2020;114(6):920–933. doi: 10.1111/mmi.14559. [DOI] [PubMed] [Google Scholar]
- 39.Terra R. Identification of Bacillus subtilis SipW as a bifunctional signal peptidase that controls surface-adhered biofilm formation. J Bacteriol. 2012;194:2781–2790. doi: 10.1128/JB.06780-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobley L. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc Natl Acad Sci U S A. 2013;110:13600–13605. doi: 10.1073/pnas.1306390110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y. Poly-γ-Glutamic acids contribute to biofilm formation and plant root colonization in selected environmental isolates of Bacillus subtilis. Front Microbiol. 2016;7:1811. doi: 10.3389/fmicb.2016.01811. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molle V. The Spo0A regulon of Bacillus subtilis. Mol Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 43.Kolodkin-Gal I. Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev. 2013;27:887–899. doi: 10.1101/gad.215244.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.López D. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci U S A. 2009;106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.López D., Gontang E.A., Kolter R. Potassium sensing histidine kinase in Bacillus subtilis. Methods Enzymol. 2010;471:229–251. doi: 10.1016/S0076-6879(10)71013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beauregard P.B. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci U S A. 2013;110:E1621–E1630. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shemesh M., Chai Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J Bacteriol. 2013;195:2747–2754. doi: 10.1128/JB.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang M. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 49.Fujita M., González-Pastor J.E., Losick R. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Branda S.S. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol. 2004;186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearns D.B. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamon M.A. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol Microbiol. 2007;66:395–399. doi: 10.1111/j.1365-2958.2007.05923.x. [DOI] [PubMed] [Google Scholar]
- 54.Cairns L.S. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Mol Microbiol. 2013;90:6–21. doi: 10.1111/mmi.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhamme D.T., Murray E.J., Stanley-Wall N.R. DegU and Spo0A jointly control transcription of two loci required for complex colony development by Bacillus subtilis. J Bacteriol. 2009;191:100–108. doi: 10.1128/JB.01236-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley N.R., Lazazzera B.A. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-gamma-dl-glutamic acid production and biofilm formation. Mol Microbiol. 2005;57:1143–1158. doi: 10.1111/j.1365-2958.2005.04746.x. [DOI] [PubMed] [Google Scholar]
- 57.Morikawa M. Biofilm formation by a Bacillus subtilis strain that produces gamma-polyglutamate. Microbiology. 2006;152:2801–2807. doi: 10.1099/mic.0.29060-0. [DOI] [PubMed] [Google Scholar]
- 58.Tan I.S., Ramamurthi K.S. Spore formation in Bacillus subtilis. Environ Microbiol Rep. 2014;6:212–225. doi: 10.1111/1758-2229.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamon M.A., Lazazzera B.A. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- 60.Piggot P., Hilbert D. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2005;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Eswaramoorthy P. The threshold level of the sensor histidine kinase KinA governs entry into sporulation in Bacillus subtilis. J Bacteriol. 2010;192:3870–3882. doi: 10.1128/JB.00466-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burbulys D., Trach K.A., Hoch J.A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- 63.Shafikhani S.H., Leighton T. AbrB and Spo0E control the proper timing of sporulation in Bacillus subtilis. Curr Microbiol. 2004;48:262–269. doi: 10.1007/s00284-003-4186-2. [DOI] [PubMed] [Google Scholar]
- 64.Whitten A.E. The structure of the KinA-Sda complex suggests an allosteric mechanism of histidine kinase inhibition. J Mol Biol. 2007;368:407–420. doi: 10.1016/j.jmb.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 65.Rowland S.L. Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol Cell. 2004;13:689–701. doi: 10.1016/s1097-2765(04)00084-x. [DOI] [PubMed] [Google Scholar]
- 66.Chai Y., Kolter R., Losick R. Reversal of an epigenetic switch governing cell chaining in Bacillus subtilis by protein instability. Mol Microbiol. 2010;78:218–229. doi: 10.1111/j.1365-2958.2010.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bressuire-Isoard C., Broussolle V., Carlin F. Sporulation environment influences spore properties in Bacillus: evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol Rev. 2018;42:614–626. doi: 10.1093/femsre/fuy021. [DOI] [PubMed] [Google Scholar]
- 68.Chen Z.M. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol. 2010;85:1353–1360. doi: 10.1007/s00253-009-2162-x. [DOI] [PubMed] [Google Scholar]
- 69.Monteiro S. Enhanced spore production of Bacillus subtilis grown in a chemically defined medium. Adv Microbiol. 2014;4:444. [Google Scholar]
- 70.Zhao Z.-M. Enhancement of Bacillus subtilis growth and sporulation by two-stage solid-state fermentation strategy. Processes. 2019;7:644. [Google Scholar]
- 71.Hashim M.A. Remediation technologies for heavy metal contaminated groundwater. J Environ Manag. 2011;92:2355–2388. doi: 10.1016/j.jenvman.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X. A sustainable bio-carrier medium for wastewater treatment: modified basalt fiber. J Clean Prod. 2019;225:472–480. [Google Scholar]
- 73.Mukherjee A.K., Bordoloi N.K. Biodegradation of benzene, toluene, and xylene (BTX) in liquid culture and in soil by Bacillus subtilis and Pseudomonas aeruginosa strains and a formulated bacterial consortium. Environ Sci Pollut Res Int. 2012;19:3380–3388. doi: 10.1007/s11356-012-0862-8. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen O.T., Ha D.D. Degradation of chlorotoluenes and chlorobenzenes by the dual-species biofilm of Comamonas testosteroni strain KT5 and Bacillus subtilis strain DKT. Ann Microbiol. 2019;69:267–277. [Google Scholar]
- 75.Fathima A., Rao J.R., Unni Nair B B. Trivalent chromium removal from tannery effluent using kaolin-supported bacterial biofilm of Bacillus sp isolated from chromium polluted soil. J Chem Technol Biotechnol. 2012;87:271–279. [Google Scholar]
- 76.Pan X. Investigation of Cr(VI) reduction and Cr(III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633. Water Res. 2014;55:21–29. doi: 10.1016/j.watres.2014.01.066. [DOI] [PubMed] [Google Scholar]
- 77.Sundar K. Bioremoval of trivalent chromium using Bacillus biofilms through continuous flow reactor. J Hazard Mater. 2011;196:44–51. doi: 10.1016/j.jhazmat.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 78.Karn S., Duan J., Jenkinson I. Book review: role of biofilms in bioremediation. Front Environ Sci, section Microbiotechnology, Ecotoxicology and Bioremediation. 2017;5 [Google Scholar]
- 79.Li C. A novel strategy for acetonitrile wastewater treatment by using a recombinant bacterium with biofilm-forming and nitrile-degrading capability. Chemosphere. 2016;161:224–232. doi: 10.1016/j.chemosphere.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Li C. Combination of a recombinant bacterium with organonitrile-degrading and biofilm-forming capability and a positively charged carrier for organonitriles removal. J Hazard Mater. 2018;353:372–380. doi: 10.1016/j.jhazmat.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 81.Schoemaker H.E., Mink D., Wubbolts M.G. Dispelling the myths--biocatalysis in industrial synthesis. Science. 2003;299:1694–1697. doi: 10.1126/science.1079237. [DOI] [PubMed] [Google Scholar]
- 82.Demirjian D.C., Morís-Varas F., Cassidy C.S. Enzymes from extremophiles. Curr Opin Chem Biol. 2001;5:144–151. doi: 10.1016/s1367-5931(00)00183-6. [DOI] [PubMed] [Google Scholar]
- 83.Qureshi N. Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Factories. 2005;4:24. doi: 10.1186/1475-2859-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Todhanakasem T. Developing microbial biofilm as a robust biocatalyst and its challenges. Biocatal Biotransform. 2017;35:86–95. [Google Scholar]
- 85.Halan B., Buehler K., Schmid A. Biofilms as living catalysts in continuous chemical syntheses. Trends Biotechnol. 2012;30:453–465. doi: 10.1016/j.tibtech.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Gross R. Microbial biofilms: new catalysts for maximizing productivity of long-term biotransformations. Biotechnol Bioeng. 2007;98:1123–1134. doi: 10.1002/bit.21547. [DOI] [PubMed] [Google Scholar]
- 87.Todhanakasem T. Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate. N Biotech. 2014;31:451–459. doi: 10.1016/j.nbt.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Mahdinia E. Modeling of vitamin K (Menaquinoe-7) fermentation by Bacillus subtilis natto in biofilm reactors. Biocatal Agric Biotechnol. 2019;17:196. 2. [Google Scholar]
- 89.Mahdinia E., Demirci A., Berenjian A. Enhanced vitamin K (Menaquinone-7) production by Bacillus subtilis natto in biofilm reactors by optimization of glucose-based medium. Curr Pharmaceut Biotechnol. 2018;19:917–924. doi: 10.2174/1389201020666181126120401. [DOI] [PubMed] [Google Scholar]
- 90.Mahdinia E., Demirci A. Biofilm reactors as a promising method for vitamin K (menaquinone-7) production. Appl Microbiol Biotechnol. 2019;103:5583–5592. doi: 10.1007/s00253-019-09913-w. [DOI] [PubMed] [Google Scholar]
- 91.Cui S. Cell membrane and electron transfer engineering for improved synthesis of menaquinone-7 in Bacillus subtilis. iScience. 2020;23:100918. doi: 10.1016/j.isci.2020.100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma Y. Metabolic engineering of the MEP pathway in Bacillus subtilis for increased biosynthesis of menaquinone-7. ACS Synth Biol. 2019;8:1620–1630. doi: 10.1021/acssynbio.9b00077. [DOI] [PubMed] [Google Scholar]
- 93.Yan L. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci Rep-Uk. 2016;6:34644. doi: 10.1038/srep34644. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen P. Optimizing bioconversion of ferulic acid to vanillin by Bacillus subtilis in the stirred packed reactor using Box-Behnken design and desirability function. Food Sci Biotechnol. 2017;26:143–152. doi: 10.1007/s10068-017-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L. UC Riverside; 2017. Spore-based designer enzyme cascade biocatalysts. [Google Scholar]
- 96.Mingmongkolchai S., Panbangred W. Display of Escherichia coli phytase on the surface of Bacillus subtilis spore using CotG as an anchor protein. Appl Biochem Biotechnol. 2019;187:838–855. doi: 10.1007/s12010-018-2855-7. [DOI] [PubMed] [Google Scholar]
- 97.Gao C. Chemoenzymatic synthesis of N-acetyl-D-neuraminic acid from N-acetyl-D-glucosamine by using the spore surface-displayed N-acetyl-D-neuraminic acid aldolase. Appl Environ Microbiol. 2011;77:7080–7083. doi: 10.1128/AEM.05601-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H. An approach for lactulose production using the CotX-mediated spore-displayed β-galactosidase as a biocatalyst. J Microbiol Biotechnol. 2016;26:1267–1277. doi: 10.4014/jmb.1602.02036. [DOI] [PubMed] [Google Scholar]
- 99.Guo Q. Enhanced d-tagatose production by spore surface-displayed l-arabinose isomerase from isolated Lactobacillus brevis PC16 and biotransformation. Bioresour Technol. 2018;247:940–946. doi: 10.1016/j.biortech.2017.09.187. [DOI] [PubMed] [Google Scholar]
- 100.He W. Production of d-allulose with d-psicose 3-epimerase expressed and displayed on the surface of Bacillus subtilis spores. J Agric Food Chem. 2016;64:7201–7207. doi: 10.1021/acs.jafc.6b03347. [DOI] [PubMed] [Google Scholar]
- 101.Li J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Potot S. Display of recombinant proteins on Bacillus subtilis spores, using a coat-associated enzyme as the carrier. Appl Environ Microbiol. 2010;76:5926–5933. doi: 10.1128/AEM.01103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang C. Engineered Bacillus subtilis biofilms as living glues. Mater Today. 2019;28:40–48. [Google Scholar]
- 104.Paz-Gómez G. Water-repellent fluoropolymer-based coatings. Coatings. 2019;9:293. [Google Scholar]
- 105.Epstein A.K. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc Natl Acad Sci U S A. 2011;108:995–1000. doi: 10.1073/pnas.1011033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angelini T. Bacillus subtilis spreads by surfing on waves of surfactant. Proc Natl Acad Sci U S A. 2009;106:18109–18113. doi: 10.1073/pnas.0905890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grumbein S. Hydrophobic properties of biofilm-enriched hybrid mortar. Adv Mater. 2016;28:8138–8143. doi: 10.1002/adma.201602123. [DOI] [PubMed] [Google Scholar]
- 108.Gilbert C., Ellis T. Biological engineered living materials: growing functional materials with genetically programmable properties. ACS Synth Biol. 2019;8:1–15. doi: 10.1021/acssynbio.8b00423. [DOI] [PubMed] [Google Scholar]
- 109.Sahin O. Physical basis for the adaptive flexibility of Bacillus spore coats. J R Soc Interface. 2012;9:3156–3160. doi: 10.1098/rsif.2012.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Setlow B. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β-type small acid-soluble proteins. J Bacteriol. 2006;188:3740–3747. doi: 10.1128/JB.00212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.González L.M., Mukhitov N., Voigt C.A. Resilient living materials built by printing bacterial spores. Nat Chem Biol. 2020;16:126–133. doi: 10.1038/s41589-019-0412-5. [DOI] [PubMed] [Google Scholar]
- 112.Chen X. Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators. Nat Nanotechnol. 2014;9:137–141. doi: 10.1038/nnano.2013.290. [DOI] [PubMed] [Google Scholar]
- 113.Ma M. Bio-inspired polymer composite actuator and generator driven by water gradients. Science. 2013;339:186–189. doi: 10.1126/science.1230262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhong C. Spores hit the spot. Nat Chem Biol. 2020;16:108–109. doi: 10.1038/s41589-019-0451-y. [DOI] [PubMed] [Google Scholar]
- 115.Lufton M. Living bacteria in thermoresponsive gel for treating fungal infections. Adv Funct Mater. 2018;28:1801581. [Google Scholar]