Abstract
Hepatotoxicity of chemotherapeutic agents such as methotrexate, oxaliplatin, and irinotecan have been well documented and characterized allowing for careful management by oncologists during administration. However, the rapid advance of the field of oncology and introduction of new classes of therapies such as small molecule inhibitors and immunotherapies have introduced new hepatotoxicity challenges and management strategies. This work is a compilation of the hepatotoxicity and recommended management of various chemotherapies and targeted agents, with a focus on the newer classes of targeted anticancer agents.
Keywords: Hepatotoxicity, liver, chemotherapy, targeted agents
Introduction
While newly developed anticancer therapies have resulted in advances in outcomes and patient survival, these improvements have been accompanied by a host of side effects such as neurotoxicity, nephrotoxicity, and cardiotoxicity [1-3]. In addition to these side effects, hepatotoxicity has also been identified as a limiting factor in the use and administration of anticancer therapies. As new, targeted cancer therapies continue to be developed, oncologists and hepatologists must work closely to monitor patients for hepatotoxicity and intervene to avoid permanent liver damage [4].
The liver plays a key role in the metabolism of a variety of drugs and toxins and thus is especially susceptible to damage induced by drugs including cytotoxic chemotherapy regimens. Additionally, drug induced liver injury can exhibit a multiplicative effect in which previous damage can feed-forward resulting in impaired drug metabolism and further toxicity. Hepatotoxicity can result from damage to structures such as the liver sinusoids, vasculature, bile ducts, and direct damage to hepatocytes themselves. Additionally, occlusion of vascular and ductal structures, toxic metabolite formation, and inflammatory cell infiltration into the liver parenchyma can induce damage. As a result, proper monitoring and strategies such as discontinuation or dose-modification of pharmacologic agents is commonly required when hepatotoxicity occurs [5-7]. General mechanisms of anticancer therapy hepatotoxicity are outlined in Figure 1.
Figure 1.
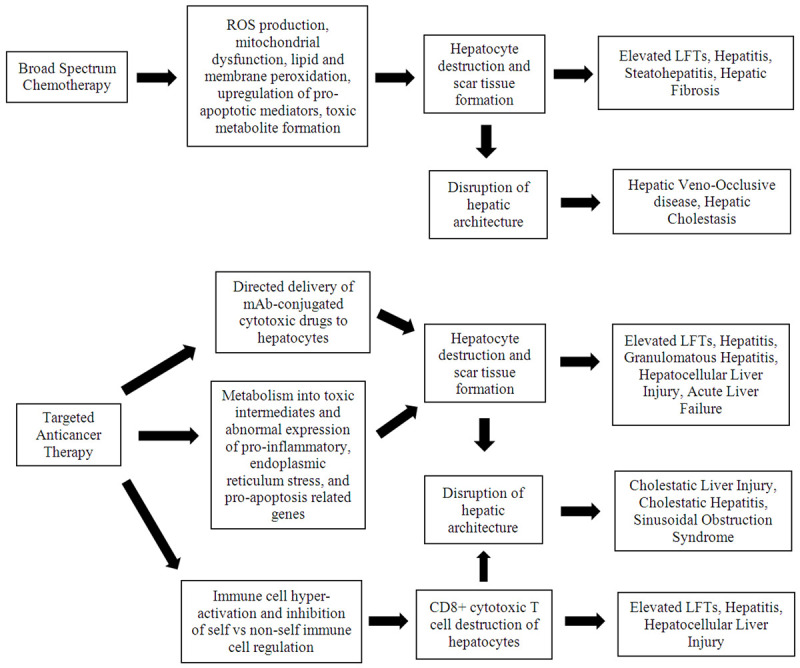
General mechanisms of anticancer treatment hepatotoxicity.
A wide variety of drugs exhibit hepatotoxicity that must be monitored for including, but not limited to, drugs such as acetaminophen, amiodarone, amoxicillin-clavulanate, and the statins. Additionally, conventional chemotherapeutic agents have well characterized hepatotoxic effects with some of the most common identified agents including methotrexate, irinotecan, and oxaliplatin [8]. Hepatotoxicity related to administration of chemotherapeutic agents includes elevations of liver function tests (LFTs), drug-induced hepatitis, veno-occlusive disease, steatohepatitis, as well as potential chronic manifestations such as fibrosis and liver failure [9-11]. Typically, chemotherapy induced hepatotoxic can be properly managed via close monitoring for elevations in LFTs that are suggestive of liver injury and dose reductions, with discontinuation of the offending agent if LFT recovery to normal levels is refractory to dose reduction [9-11]. Further complications to treatment strategy and management results from the complex nature of cancer patients, as many present with additional conditions including previous liver injury. In cases in which liver function is already compromised, care must be taken to properly adjust drug dosages and to tailor therapy regimens correspondingly [12].
Special considerations for hepatotoxicity of nonspecific chemotherapy agents targeting either primary or metastatic liver tumors require mention, including cases in which radiotherapy is used in addition to adjunctive therapy as well as the treatment of malignancies with hepatic metastasis such as colorectal carcinoma (CRC). Radiation therapy has been employed to target tumors within the liver using both external irradiation and radioembolization, but these techniques have been associated with either direct hepatotoxicity or a synergistic increase in hepatotoxicity of adjuvant chemotherapy regimens. Agents that have been shown to cause increased hepatotoxicity in conjunction with radiation therapy include vincristine, doxorubicin, and dactinomycin [10,11,13]. Additionally, anticancer therapy hepatotoxicity in cases of liver metastasis from CRC tumors are of special concern due to the potential for hepatectomy-limiting liver damage to occur. Adjunctive treatments used before surgical resection of CRC and hepatic metastasis that have been shown to cause hepatotoxicity include 5-flourocuracil, irinotecan, and oxaliplatin [14,15]. Special care must be taken in these circumstances to avoid surgery limiting hepatotoxicity and to intervene with dose reduction or discontinuation if initial signs of hepatotoxicity are detected [16,17].
Since hepatotoxicity of established chemotherapies have been well described, this review will briefly touch on their associated liver toxicities as well as the epidemiology of cancer therapy associated liver damage and monitoring strategies for hepatotoxicity. Classes of chemotherapies that cause hepatotoxicity include the antitumor antibiotics, alkylating agents, platinum agents, antimetabolites, antimicrotubular agents, and topoisomerase inhibitors. The rest of this review will place more focus on the hepatotoxicity of new classes of anticancer therapies. These targeted agents include anti-HER-2 therapies, targeted small molecule inhibitors such as VEGF and tyrosine kinase inhibitors, immune checkpoint inhibitors (ICI), and chimeric antigen receptor T (CAR-T) cells. Tables 1 and 2 provide an overview of the hepatotoxic effects associated with chemotherapy and targeted agents, respectively, as well as their management.
Table 1.
List of chemotherapy agents which cause hepatotoxicity
| Agent | Hepatotoxic Effect | Management | Ref |
|---|---|---|---|
| Antitumor Antibiotics | |||
| 1. Dactinomycin | Elevated LFTs | • Elevated ALT/AST - Decrease dose, D/C agent if refractory | [9,10,21] |
| 2. Doxorubicin | Veno-occlusive disease | • Veno-occlusive disease - D/C agent, supportive care for complications of portal hypertension | |
| 3. Mitomycin | |||
| Alkylating agents | |||
| 1. Busulfan | Elevated LFTs | • Elevated LFTs - Decrease dose, D/C agent if refractory | [9-11] |
| 2. Melphalan | Veno-occlusive disease | • Veno-occlusive disease - D/C agent, supportive care for complications of portal hypertension | |
| 3. Cyclophosphamide | |||
| Platinum based agents | |||
| 1. Oxaliplatin | Elevated LFTs | • Elevated LFTs - decrease dose, D/C agent | [4,10,38] |
| 2. Cisplatin | Steatohepatitis | • Steatohepatitis - D/C agent | |
| 3. Carboplatin | Veno-occlusive disease | • Veno-occlusive disease - D/C agent, supportive care for complications of portal hypertension | |
| Antimetabolites | |||
| 1. Methotrexate | Elevated LFTs | • Elevated LFTs - decrease dose, D/C agent if refractory | [9-11,26,88] |
| 2. 6-Mercaptopurine | Hepatitis | • Hepatitis - decrease dose, D/C agent if refractory; corticosteroids | |
| 3. Azathioprine | Hepatic Cholestasis | • Hepatic Cholestasis - D/C agent | |
| 4. Cytarabine | Hepatic Fibrosis | • Hepatic Fibrosis - D/C agent | |
| 5. Fluorodeoxyuridine | |||
| 6. Gemcitabine | |||
| Antimicrotubular Agents | |||
| 1. Paclitaxel | Elevated LFTs | • Elevated LFTs - decrease dose, D/C agent if refractory | [9-11,26] |
| 2. Docetaxel | Hepatitis | • Hepatitis - decrease dose, D/C agent if refractory; corticosteroids | |
| 3. Vincristine | |||
| 4. Vinblastine | |||
| Topoisomerase Inhibitors | |||
| 1. Irinotecan | Elevated LFTs | • Elevated LFTs - decrease dose, D/C agent if refractory | [4,9-11] |
| 2. Etoposide | Steatohepatitis | • Steatohepatitis - D/C agent |
Table 2.
List of targeted agents which cause hepatotoxicity
| Agent | Hepatotoxic Effect | Management | Reference |
|---|---|---|---|
| HER-2 Inhibitors | |||
| 1. Trastuzumab | Elevated LFTs | • Elevated LFTs - D/C agent | [45-47,52,55] |
| 2. Lapatinib | Sinusoidal Obstruction Syndrome | • Sinusoidal Obstruction Syndrome - D/C agent, manage complications of portal hypertension | |
| Hepatitis | • Hepatitis - D/C agent | ||
| Small molecule TKIs and VEGF-inhibitors | |||
| 1. Imatinib | Elevated LFTs | • Elevated LFTs - decrease dose, D/C agent if refractory | [10,11,58-62,89-92] |
| 2. Vemurafenib | Hepatitis | • Hepatitis - D/C agent | |
| 3. Erlotinib | Cholestatic Hepatitis | • Cholestatic Hepatitis - D/C agent | |
| 4. Gefitinib | Granulomatous Hepatitis | • Granulomatous Hepatitis - D/C agent | |
| 5. Crizotinib | Hepatocellular Liver Injury | • Hepatocellular Liver Injury - D/C agent, corticosteroids | |
| 6. Sorafenib | Acute Liver Failure | • Acute Liver Failure - D/C agent, corticosteroids; liver transplant | |
| 7. Bosutinib | |||
| 8. Lapatinib | |||
| 9. Nilotinib | |||
| 10. Pazopanib | |||
| 11. Regorafenib | |||
| 12. Sunitinib | |||
| Checkpoint Inhibitors | |||
| 1. Nivolumab | Elevated LFTs | • Elevated LFTs - monitor closely; if severe D/C agent, initiate corticosteroids | [74-78,80,90,93] |
| 2. Pembrolizumab | Hepatitis | • Hepatitis - corticosteroids, D/C agent, immunosuppressants if severe | |
| 3. Atezolizumab | Cholestatic Liver Injury | • Cholestatic Liver Injury - D/C agent, corticosteroids, ursodeoxycholic acid | |
| 4. Darvalumab | Hepatocellular Liver Injury | • Hepatocellular Liver Injury - D/C agent, corticosteroids, antithymocyte globulin, mycophenolate mofetil | |
| 5. Avelumab | |||
| 6. Tremelimumab | |||
| 7. Ipilimumab | |||
| CAR-T Cells | Elevated LFTs | • Elevated LFTs - D/C agent; corticosteroids if severe | [84,87] |
Epidemiology of cancer therapy hepatotoxicity
The rate of drug associated liver injury within the general population is estimated at about 15.4-23.3 cases per 100,000 people based on a prospective cohort study performed in Iceland [18]. More recent study of a retrospective cohort in mainland China reported a drug associated liver injury rate of between 20.86-26.74 cases per 100,000, with the population level differences potentially being attributed to diet and the use of traditional Chinese medicines [19]. While these general population rates provide a baseline picture of hepatotoxicity incidence, specific drugs exhibit considerably higher hepatotoxicity rates, for example, amoxicillin-clavulanate therapy has an estimated rate of liver injury of 8-22% between different studies [20]. In examples of nonspecific chemotherapeutic agents, study of a cohort of breast cancer patients receiving doxorubicin found a hepatotoxicity rate of 30.4% while study of head and neck squamous cell carcinoma patients receiving cisplatin and docetaxel found a hepatotoxicity rate of 22.2% [21,22]. Hepatotoxicity rates for newer, targeted therapies have been monitored in clinical trials as new treatments have been developed. ICIs targeting CTLA-4, PD-1, and PD-L1 have a hepatotoxicity rate between 5-10% in patients receiving treatment, usually characterized by elevated LFTs [23]. Additionally, small molecule vascular endothelial growth factor (VEGF) inhibitors and tyrosine kinase inhibitors (TKIs) have reported all-grade hepatotoxicity rates of between 6.6-15.5% and 25-35%, respectively [24,25]. These values highlight the need for close monitoring for treatment related hepatotoxicity in patients receiving anticancer therapy.
Monitoring for hepatotoxicity
Hepatotoxicity represents a relatively common and potentially serious side effect of anticancer therapies. Hepatotoxicity can manifest in many different ways ranging from acute, transient elevations in LFTs such as bilirubin or liver enzymes to long term complications such as cirrhosis or liver failure if liver damage progresses unnoticed [7]. Thus, proper recognition of patients at risk for hepatotoxicity before anticancer treatment is started and continued evaluation of patients receiving treatment for liver injury by their oncologist is essential. As previously mentioned, the liver plays a key role in the metabolism of drugs and their potentially toxic metabolites, therefore, the presence of existing liver damage or dysfunction calls for careful adjustment of drug dosing as well as adjustment of the drugs within a given patients therapy regimen [12]. LFTs represent the primary baseline measure of liver function and health in patients receiving anticancer therapy, and elevations in LFTs represent a majority of therapy-related hepatotoxicity [11,26]. In a prospective study exploring baseline liver function in patients with unresectable hepatocellular carcinoma who were treated with lenvatinib, patients with higher liver function scores (based on albumin-bilirubin grading and Child-Pugh scoring) less adverse effects and a lower rate of treatment discontinuation due to adverse events [27]. These results suggest that baseline liver function evaluation represents a prospective measurement that can predict the likelihood of hepatotoxicity in patients receiving anticancer treatment. In the event of elevated LFTs suggestive of hepatotoxicity, other evaluation strategies including liver biopsy, ultrasound, and magnetic resonance imaging can be employed to visualize structural damage. For example, magnetic resonance imaging has been employed to visualize potential liver damage in patients receiving oxaliplatin therapy prior to surgery for metastatic CRC [28]. Looking to the future, studies have begun to evaluate new blood biomarkers that can indicate drug induced liver damage, with one such study identifying that levels glutamate dehydrogenase, caspase cleaved K18, osteopontin, and macrophage colony-stimulating factor receptor represented potential candidate biomarkers for drug induced liver damage evaluation [29].
Risk factors for hepatotoxicity
The risk of drug therapy related hepatotoxicity can be altered by a variety of patient specific factors. For example, factors that increase risk of acetaminophen related hepatotoxicity include alcohol use, the parallel use of other hepatotoxicity drugs, and genetic polymorphisms in genes such as glutathione synthase [30,31]. As the hepatotoxicity of cancer related treatments has been further characterized, more focus has moved to the identification of patient specific risk factors that contribute to increased risk of toxicity especially in the more recently developed targeted agents. A retrospective study of melanoma patients who received immune checkpoint inhibitor therapy found that concurrent infection and subsequent antibiotic treatment represented potential contributors to increased hepatotoxicity risk [32]. The tyrosine kinase inhibitors represent another class of therapies that patient specific factors that increased hepatotoxicity rates have been explored for. A retrospective study of non-small cell lung cancer patients receiving crizitonib found that the use of proton pump inhibitors as well as the presence of liver disease or hepatitis B virus infection were correlated with higher hepatotoxicity rates [33]. Similar results were seen with patients taking lapatinib, erlotinib, and imatinib with patients receiving proton pump inhibitors having higher rate of hepatotoxicity [22,34,35]. Additionally, other factors that increased hepatotoxicity rates in patients receiving these treatments included CYP3A4 inducers in patients receiving lapatinib or erlotinib and low patient body weight in patients receiving imatinib.
Hepatotoxicity of broad-spectrum chemotherapy classes
Broad spectrum chemotherapy has traditionally represented the backbone of anticancer therapy regimens. Chemotherapy typically induces cellular damage that results in impaired cell division or apoptosis of rapidly dividing cells. The nonselective characteristics of traditional chemotherapeutic agents result in off target adverse effects including, but not limited to, hepatotoxicity. The hepatotoxic effects of traditional chemotherapies include elevation of LFTs, hepatitis, cholestasis, steatohepatitis, and hepatic veno-occlusive disease. A wide variety of classes of chemotherapies have been shown to cause hepatotoxicity including the antitumor antibiotics, alkylating agents, platinum agents, antimetabolites, antimicrotubular agents, and topoisomerase inhibitors [9-11]. The management of chemotherapy induced hepatotoxicity has primarily employed the use of decreased medication doses in the face of hepatotoxicity or the discontinuation of the offending agent if the liver injury remains refractory to dose decreases. While traditional chemotherapy agents have been extensively reviewed (see refs. [9-11]), new literature continues to study and report cases of hepatotoxicity due to chemotherapy.
As chemotherapeutic agents continue to be further studied, mechanisms of their off-target toxicities have been of interest to better understand and avoid severe side effects of therapy. For example, research exploring the mechanism of methotrexate related hepatotoxicity identified the activation of inflammatory pathways and cytokines, upregulation of pro-apoptotic mediators, and reactive oxygen species (ROS) formation as contributing factors to liver damage. Additionally, the group found that 18β-glycyrrhetinic acid, a potentially hepatoprotective molecule, was able to diminish the hepatotoxic mechanisms associated with methotrexate [36]. Another study exploring the mechanisms of doxorubicin related hepatotoxicity found that ROS production, membrane lipid peroxidation, upregulation of pro-apoptotic genes such as Bax, and impaired mitochondrial energy metabolism all contributed to liver damage [37]. As the mechanisms of chemotherapy related hepatotoxicity continue to be elucidated, new therapies to attenuate these deleterious hepatic effects can be explored and developed.
Recently published case reports of chemotherapy related hepatotoxicity include toxicity related to oxaliplatin treatment as well as a cisplatin and 5-FU regimen [38,39]. The patient experiencing oxaliplatin hepatotoxicity presented with esophagogastric varices secondary to veno-occlusive disease during a follow up visit 3.5 years after receiving oxaliplatin therapy for liver metastasis [38]. The case report of a patient receiving cisplatin and 5-FU outlines the onset of severe hepatitis that was preceded by an elevation in LFTs and was successfully treated with discontinuation of the chemotherapy regimen and initiation of corticosteroids (dexamethasone) [39]. Both of these cases highlight the rare but potentially severe hepatotoxicity that can occur due to chemotherapy and the need to closely monitor the hepatic function of patients receiving chemotherapy as well as continued monitoring for long-term sequela of chemotherapy associated hepatotoxicity. Management strategies in the face of chemotherapy associated hepatotoxicity can be found in Table 1 and primarily involve dosage decreases or discontinuation of the hepatotoxic agent.
Hepatotoxicity of targeted agents
HER-2 inhibitors
HER-2 or Human Epidermal Growth Factor Receptor 2 is a proto-oncogene that is mutated in between 15-20% of breast cancer tumors [40]. Amplification of HER-2 signaling promotes oncogenic pathways including cell growth, proliferation, and cell survival via the PI3K-AKT-mTOR-RAS-MAPK signaling pathway [41]. Trastuzumab is an anti-HER-2 antibody that can either be used as a HER-2 singling antagonist or can be conjugated to cytotoxic molecules such as emtansine to directly deliver chemotherapy to HER-2 positive cancer cells for antitumor effects [42]. Targeted therapies against HER-2 such as trastuzumab/trastuzumab emtansine have contributed to improved outcomes in HER-2 positive breast cancers and now are being evaluated for activity against other HER-2 positive cancers [43]. While trastuzumab continues to be widely used in the treatment of HER-2 positive tumors, it also can cause hepatotoxicity via direct damage to hepatocytes and subsequent upregulation of TNF-alpha signaling that further contributes to hepatocyte damage [44].
Case reports of hepatotoxicity secondary to trastuzumab continue to be published, including a patient who experienced elevated LFTs six months after starting trastuzumab, a patient who experienced recurrent LFT elevations with trastuzumab rechallenge, and two patients who experienced long term hepatic veno-occlusive disease years after treatment [45-47]. In the first two cases in which the patients experienced increased LFTs, discontinuation of trastuzumab administration was sufficient for the LFT levels to return to normal [45,46]. Treatment of the patients experiencing hepatic veno-occlusive disease involved management and treatment of sequela of portal hypertension including esophageal varices and hepatic encephalopathy [47].
Data from clinical trials has allowed for estimation of trastuzumab/trastuzumab emtansine hepatotoxicity rates. A prospective, phase II study of Japanese breast cancer patients receiving trastuzumab emtansine found that 60.3% of patients experienced any grade hepatotoxicity with AST/ALT elevations as the most commonly occurring hepatotoxic event. However, the hepatotoxicity for nearly all the patients was transient with only one patient requiring a dose decrease due to liver damage [48]. Additional data from a meta-analysis of seven trials of breast cancer patients found that those receiving trastuzumab emtansine had a relative risk of 3.24 for experiencing all-grade AST/ALT elevation vs control treatment [49]. Finally, a small trail of the treatment of HER-2 positive non-small cell lung cancer (NSCLC) patients with trastuzumab emtansine reported a rate of 3 out of 15 or 20% patients experiencing hepatotoxicity [50]. Taken together, these findings call for careful monitoring of patients receiving trastuzumab therapy so that potentially serious hepatotoxicity can be properly managed before long-term liver injury occurs.
Lapatinib represents another member of the anti-HER-2 family of targeted therapies against HER-2 positive breast cancer that is additionally being explored in the treatment of other HER-2 positive cancers, similarly to trastuzumab [51]. Hepatotoxicity related to lapatinib treatment occurs similarly to that of trastuzumab, but other work found an additional mechanism of liver injury in which lapatinib increases the accumulation of combination chemotherapy via inhibition of ATP-binding cassette transporters [52]. Lapatinib induced hepatotoxicity has been associated with HLA-DQA1*02:01 and HLA-DRB1*07:01 and a report outlining a case of lapatinib induced hepatitis that responded to therapy discontinuation exhibits the potential for lapatinib associated hepatotoxicity [53-55].
Small molecule TKIs and VEGF inhibitors
Small molecule TKIs and VEGF inhibitors represent a broad category of targeted anticancer therapies used to treat wide variety of cancer types. These drugs are designed to target specific signaling molecules or cell receptors to block oncogenic pathways such as angiogenesis, growth signaling, and cell-cycle amplification and allow for patient-tailored treatment based on the mutational profile of their cancer [56,57]. While these drugs have vastly improved patient outcomes, they also are accompanied by a host of side effects including hepatotoxicity. As previously mentioned, VEGF inhibitors have a reported hepatotoxicity rate between 6.6-15.5% while TKIs have a reported hepatotoxicity rate between 25-35% highlighting the need for close monitoring and intervention by clinicians administering these treatments [24,25].
A number of case reports providing examples of TKI and VEGF inhibitor associated hepatotoxicity and their management have been published, including severe cases of fatal toxicity. Sorafenib, a Raf/PDGFβ/VEGF inhibitor, has been associated with hepatitis, with one case report describing progression from hepatitis to ultimately fatal hepato-renal failure in a renal cell carcinoma patient who was unable to be a candidate for liver transplant [58]. Hepatotoxicity associated with the VEGF inhibitor Pazopanib was reported in a case outlining two patients, each of whom experienced cholestatic hepatitis, with one of the cases being fatal [59]. Vemurafenib, a commonly used BRAF inhibitor, has been associated with hepatotoxicity including a case of granulomatous hepatitis that progressed to chronic cholestasis even with treatment discontinuation [60]. Imatinib is another commonly used TKI that has activity against Philadelphia chromosome positive chronic myeloid leukemia, however it has been associated with potentially severe hepatotoxicity. One recent case reported a patient who experienced hepatocellular liver injury that was refractory to treatment discontinuation but did resolve with treatment with methylprednisolone [61]. These cases provide examples of the potentially severe nature of hepatotoxicity associated with TKIs and VEGF inhibitors and the need for close monitoring of patients who are receiving these therapies so that the proper management steps such as dose reduction and discontinuation of the offending agent can be initiated, as seen in Table 2.
Another interesting phenomenon that has emerged with the large number of TKIs and VEGF inhibitors that have been developed is that therapies that inhibit the same signaling pathway may not cause liver injury upon rechallenge with a new agent in a patient who has previously experienced hepatotoxicity. For example, two separate case reports outlined the successful transition and treatment of patients who experienced and recovered from gefitinib associated hepatotoxicity to erlotinib, another EGFR inhibitor that blocks the same pathway as gefitinib [62,63]. Subsequent case reports have confirmed this EGFR inhibitor treatment strategy with successful treatment of patients with afatinib after both gefitinib and erlotinib hepatotoxicity occurred [64,65]. The principle of successful rechallenge with a new agent in the face of treatment related hepatotoxicity due to TKIs and VEGF inhibitors appears applicable to treatments targeting other signaling pathways as well, with cases of successful transitions of therapy from sunitinib to sorafenib and imatinib to dasatinib reported in the literature [66,67].
Due to the widespread and potentially serious nature of hepatotoxicity related to TKIs and VEGF inhibitors, more recent studies have attempted to outline the hepatotoxicity rates of specific agents as well as factors that increase risk or contributed to hepatotoxicity of these agents. Hepatotoxicity associated with individual TKI agents is quite common, with studies finding hepatotoxicity rates of 39.6% for gefitinib and up to 32% for regorafenib, with a second study of regorafenib reporting a fatal hepatotoxicity rate of 0.33% [68-70]. A retrospective cohort study of the hepatotoxicity of individual VEGF inhibitors found hepatotoxicity rates of up to 41.5% for pazopanib, 49.4% for bevacizumab, 37.9% for sorafenib, and 42.6% for sunitinib [71]. Further study evaluating the specific hepatotoxicity rates and phenotypes of TKI and VEGF inhibitors will allow oncologists to better tailor treatment for patients and quickly identify liver injury when it occurs.
Looking to the future, new research into the hepatotoxicity of TKIs and VEGF inhibitors is exploring the various factors that potentially contribute to liver injury or susceptibility to toxicity. A retrospective analysis examining a cohort of patients receiving erlotinib found that hepatotoxicity was increased 2.7-fold in patients who were also receiving CYP3A4 inducers, 3.5-fold in patients using H2-antagonists, and that the presence of liver metastasis and increased age were significant hepatotoxicity risk factors [34]. A similar retrospective analysis exploring hepatotoxicity of imatinib found that the incidence of hepatotoxicity was increased 3.8-fold in patients receiving H2-antagonists, 2.3-fold in patients receiving high dose imatinib therapy (> 400 mg), and 8-fold and 5.2-fold for patients with preexisting liver disease or latent hepatitis B virus, respectively [35]. Another recently published multi-center retrospective analysis examining several TKIs found similar results that H2-antagonists and CYP3A4 inducers increase hepatotoxicity, indicating that these factors should be avoided if possible in patients receiving TKI therapy to avoid increased risk of liver injury [25]. In addition to identifying exogenous risk factors such as pharmacologic agents that can contribute to increased hepatotoxicity risk of TKIs and VEGF inhibitors, risk stratification based on endogenous factors such as genetic profiles holds promise as personalized medicine continues to develop. One such study exploring the pharmacogenomics of a prospective patient cohort found that single nucleotide polymorphisms in genes coding for EGFR, CYP3A5, and cytochrome p450 reductase were correlated with hepatotoxicity [72]. Further studies into the genetic risk factors of TKIs and VEGF inhibitors will provide the background needed to develop genetic risk factor panels that used to tailor anticancer treatment based on a patient’s genotype.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs) represent one of the most exciting recent developments in anticancer therapy. These therapies block regulatory pathways that normally attenuate immune function and thus disinhibit immune cells to destroy cancer cells. The two current pathways that ICIs target include CTLA-4 and PD-1 signaling, however, this disinhibition of immune function brings about side effects due to autoimmune damage to self-tissues including the liver. Histological study of liver biopsy samples in patients experiencing ICI associated hepatotoxicity found a predominantly lobular pattern of hepatitis and notable infiltration of CD3+ and CD8+ T cells into the liver parenchyma in the samples, supporting a mechanism of injury due to autoimmune attack of the liver [73]. These findings provide support for the common management strategy of ICI related hepatotoxicity, which includes discontinuation of the offending agent as well as corticosteroids to attenuate immune cell activity, with the use of methylprednisolone and mycophenolate mofetil advised in the case of refractory toxicity, as outlined in Table 2 [74,75].
Case reports outlining hepatotoxicity related to ICI therapy have been widely published and have provided additional insight to the management of ICI-related hepatotoxicity. One report of a patient receiving ipilimumab, an anti-CTLA-4 monoclonal antibody, outlined a patient who suffered from hepatocellular injury that was successfully treated with a triple immunosuppressant therapy of methylprednisolone, antithymocyte globulin, and mycophenolate mofetil [76]. Additional cases of hepatotoxicity related to agents targeting the PD-1/PD-L1 immune checkpoint include a case of hepatitis in a patient receiving long term treatment with nivolumab and a case of cholestatic liver injury in a patient receiving pembrolizumab. In both cases, the patients were successfully treated with discontinuation of the ICI agent and systemic corticosteroids [77,78]. While ICI related hepatotoxicity has the potential to be successfully treated, fatal cases have occurred including a recently published case of fatal cholestatic hepatitis in a liver transplant patient with a history of hepatocellular carcinoma who was receiving nivolumab [79]. The serious nature of ICI related hepatotoxicity and the relative risk of their hepatotoxicity vs chemotherapy, estimated to be about 2.14 for hepatitis, provides a strong argument for further study of ICI therapy to determine risk factors that contribute to predisposition toward ICI therapy hepatotoxicity [80]. Additionally, the development of preventative measures that can balance between preventing autoimmune toxicity while maintaining antitumor activity of ICIs can diminish the side effect profile of these highly effective therapies.
CAR-T cells
CAR-T cells are another exciting new development in the field of oncology that have provided a new class of treatments for hematologic cancers including lymphoma and leukemia. CAR-T cell therapy harnesses the immune system similarly to ICI therapy, but unlike the inhibition releasing activity of ICIs, CAR-T cells are patient derived T cells that are engineered to target specific markers harbored on tumor cells [81]. CAR-T cells are being studied for use against both hematologic and solid malignancies and will become more widely used as they continue to be developed and improved [82]. Similar to ICI therapy, CAR-T cells can cause a variety of serious side effects with the most common being cytokine release syndrome [83]. Additionally, and of note for this review, CAR-T cells have been shown to cause hepatotoxicity in the form of elevated LFTs, however, these effects are typically mild and usually will spontaneously improve [84]. CAR-T cells have been studied in patients with hepatitis B and C, and the results indicate that the therapy is safe to use in these patients, without a significant risk of hepatotoxicity [85,86]. While most data suggests that hepatotoxicity due to CAR-T cells is not a cause for concern, especially compared to other adverse effects such as cytokine release syndrome, more work is needed to characterize their hepatotoxicity profile. One prospective study of treatment of renal cell carcinoma patients with CAR-T cells reported that 4 of 12 patients experienced LFT elevations that necessitated treatment discontinuation and corticosteroid administration, with liver biopsy exhibiting CAR-T cell infiltration into the hepatic parenchyma due to hepatic expression of the renal cell carcinoma antigen targeted by the engineered cells [87]. This study highlights the need to continue to evaluate the potential for CAR-T cell induced hepatotoxicity, especially as further development focuses on targeting solid tumors that are more likely to harbor antigenic targets that are shared with hepatic cells.
Conclusions
In conclusion, hepatotoxicity represents a common clinical manifestation that is associated with a variety of anticancer therapies. The inherent toxicity of anticancer therapies requires oncologists to maintain a broad awareness of their effects on the body, including the liver. As novel anticancer therapies are developed, especially targeted agents, clinicians must remain informed their new side effects, including hepatotoxicity. This review provides an overview of the hepatotoxicity of anticancer therapies including chemotherapy and targeted agents, as well as their management strategies. The use of proper monitoring strategies, rapid judgment, and appropriate decision making can allow oncologists to recognize the manifestation of anticancer therapy hepatotoxicity and intervene accordingly.
Disclosure of conflict of interest
None.
References
- 1.Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat Rev Clin Oncol. 2016;13:92–105. doi: 10.1038/nrclinonc.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Małyszko J, Kozłowska K, Kozłowski L, Małyszko J. Nephrotoxicity of anticancer treatment. Nephrol Dial Transplant. 2017;32:924–936. doi: 10.1093/ndt/gfw338. [DOI] [PubMed] [Google Scholar]
- 4.Choti MA. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Ann Surg Oncol. 2009;16:2391–4. doi: 10.1245/s10434-009-0512-7. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 6.Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914–928. e1. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoofnagle JH, Björnsson ES. Drug-induced liver injury-types and phenotypes. New N Engl J Med. 2019;381:264–273. doi: 10.1056/NEJMra1816149. [DOI] [PubMed] [Google Scholar]
- 8.Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. doi: 10.3390/ijms17020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–76. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 10.Floyd J, Mirza I, Sachs B, Perry MC. Hepatotoxicity of chemotherapy. Semin Oncol. 2006;33:50–67. doi: 10.1053/j.seminoncol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Grigorian A, O’Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol. 2014;2:95–102. doi: 10.14218/JCTH.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Periáñez-Párraga L, Martínez-López I, Ventayol-Bosch P, Puigventós-Latorre F, Delgado-Sánchez O. Drug dosage recommendations in patients with chronic liver disease. Rev Esp Enferm Dig. 2012;104:165–84. doi: 10.4321/s1130-01082012000400002. [DOI] [PubMed] [Google Scholar]
- 13.Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. doi: 10.1155/2013/815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun YS, Laurent A, Maru D, Vauthey JN. Management of chemotherapy-associated hepatotoxicity in colorectal liver metastases. Lancet Oncol. 2009;10:278–86. doi: 10.1016/S1470-2045(09)70064-6. [DOI] [PubMed] [Google Scholar]
- 15.Robinson SM, Wilson CH, Burt AD, Manas DM, White SA. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–99. doi: 10.1245/s10434-012-2438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWhirter D, Kitteringham N, Jones RP, Malik H, Park K, Palmer D. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: a review of mechanisms and outcomes. Crit Rev Oncol Hematol. 2013;88:404–15. doi: 10.1016/j.critrevonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Gangi A, Lu SC. Chemotherapy-associated liver injury in colorectal cancer. Therap Adv Gastroenterol. 2020;13:1756284820924194. doi: 10.1177/1756284820924194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of iceland. Gastroenterology. 2013;144:1419–1425. e3. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, Xu J, Niu J, Liu J, Watkins PB, Aithal GP, Andrade RJ, Dou X, Yao L, Lv F, Wang Q, Li Y, Zhou X, Zhang Y, Zong P, Wan B, Zou Z, Yang D, Nie Y, Li D, Wang Y, Han X, Zhuang H, Mao Y, Chen C. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156:2230–2241. e11. doi: 10.1053/j.gastro.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. doi: 10.1016/j.mayocp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Damodar G, Smitha T, Gopinath S, Vijayakumar S, Rao Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection Doxorubicin. Ann Med Health Sci Res. 2014;4:74–9. doi: 10.4103/2141-9248.126619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon JY, Han JM, Seo I, Gwak HS. Risk factors associated with the incidence and time to onset of lapatinib-induced hepatotoxicity. Breast Cancer Res Treat. 2019;178:239–244. doi: 10.1007/s10549-019-05382-x. [DOI] [PubMed] [Google Scholar]
- 23.Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 24.Ghatalia P, Je Y, Mouallem NE, Nguyen PL, Trinh QD, Sonpavde G, Choueiri TK. Hepatotoxicity with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2015;93:257–76. doi: 10.1016/j.critrevonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Han JM, Han HW, Yee J, Kim MK, Moon JY, Cho S, Jung D, Cho YS, Seo I, Kim JY, Gwak HS. Factors affecting high-grade hepatotoxicity of tyrosine kinase inhibitors in cancer patients: a multi-center observational study. Eur J Clin Pharmacol. 2020;76:1183–1191. doi: 10.1007/s00228-020-02897-x. [DOI] [PubMed] [Google Scholar]
- 26.Andrade RJ, Aithal GP, Björnsson ES, Kaplowitz N, Kullak-Ublick GA, Larrey D, Karlsen TH. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70:1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Ueshima K, Nishida N, Hagiwara S, Aoki T, Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Hiraoka A, Johnson P, Kudo M. Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: a multicenter study. Cancers (Basel) 2019;11:952. doi: 10.3390/cancers11070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ünal E, Karaosmanoğlu AD, Ozmen MN, Akata D, Karcaaltincaba M. Hepatobiliary phase liver MR imaging findings after Oxaliplatin-based chemotherapy in cancer patients. Abdom Radiol (NY) 2018;43:2321–2328. doi: 10.1007/s00261-018-1482-7. [DOI] [PubMed] [Google Scholar]
- 29.Church RJ, Kullak-Ublick GA, Aubrecht J, Bonkovsky HL, Chalasani N, Fontana RJ, Goepfert JC, Hackman F, King NMP, Kirby S, Kirby P, Marcinak J, Ormarsdottir S, Schomaker SJ, Schuppe-Koistinen I, Wolenski F, Arber N, Merz M, Sauer JM, Andrade RJ, van Bömmel F, Poynard T, Watkins PB. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology. 2019;69:760–773. doi: 10.1002/hep.29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17:587–607. viii. doi: 10.1016/j.cld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Larrey D, Pageaux GP. Genetic predisposition to drug-induced hepatotoxicity. J Hepatol. 1997;26(Suppl 2):12–21. doi: 10.1016/s0168-8278(97)80492-8. [DOI] [PubMed] [Google Scholar]
- 32.Romanski NA, Holmstroem RB, Ellebaek E, Svane IM. Characterization of risk factors and efficacy of medical management of immune-related hepatotoxicity in real-world patients with metastatic melanoma treated with immune checkpoint inhibitors. Eur J Cancer. 2020;130:211–218. doi: 10.1016/j.ejca.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Jung D, Han JM, Yee J, Kim JY, Gwak HS. Factors affecting crizotinib-induced hepatotoxicity in non-small cell lung cancer patients. Med Oncol. 2018;35:154. doi: 10.1007/s12032-018-1213-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim MK, Yee J, Cho YS, Jang HW, Han JM, Gwak HS. Risk factors for erlotinib-induced hepatotoxicity: a retrospective follow-up study. BMC Cancer. 2018;18:988. doi: 10.1186/s12885-018-4891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han JM, Yee J, Cho YS, Gwak HS. Factors influencing imatinib-induced hepatotoxicity. Cancer Res Treat. 2020;52:181–188. doi: 10.4143/crt.2019.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud AM, Hussein OE, Hozayen WG, Abd El-Twab SM. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of PPARγ and Nrf2: protective effect of 18β-Glycyrrhetinic acid. Chem Biol Interact. 2017;270:59–72. doi: 10.1016/j.cbi.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Prasanna PL, Renu K, Valsala Gopalakrishnan A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020;250:117599. doi: 10.1016/j.lfs.2020.117599. [DOI] [PubMed] [Google Scholar]
- 38.Shigefuku R, Watanabe T, Mizukami T, Matsunaga K, Hattori N, Ehira T, Suzuki T, Nakano H, Sato Y, Matsuo Y, Nakahara K, Ikeda H, Matsumoto N, Tsuda T, Katayama M, Koizumi S, Okuse C, Suzuki M, Otsubo T, Nakajima TE, Yasuda H, Itoh F. Esophagogastric varices were diagnosed in a non-cirrhotic liver case during long-term follow-up after oxaliplatin-based chemotherapy. Clin J Gastroenterol. 2018;11:487–492. doi: 10.1007/s12328-018-0873-1. [DOI] [PubMed] [Google Scholar]
- 39.Yaegashi A, Yoshida K, Suzuki N, Shimada I, Tani Y, Saijo Y, Toyama A. A case of severe hepatotoxicity induced by cisplatin and 5-fluorouracil. Int Cancer Conf J. 2020;9:24–27. doi: 10.1007/s13691-019-00394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014;21:100–7. doi: 10.1097/PAP.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 41.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 42.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 44.Yan H, Endo Y, Shen Y, Rotstein D, Dokmanovic M, Mohan N, Mukhopadhyay P, Gao B, Pacher P, Wu WJ. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther. 2016;15:480–90. doi: 10.1158/1535-7163.MCT-15-0580. [DOI] [PubMed] [Google Scholar]
- 45.Vucicevic D, Carey EJ, Karlin NJ. Trastuzumab-induced hepatotoxicity: a case report. Breast Care (Basel) 2013;8:146–8. doi: 10.1159/000346844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizuna K, Ninomiya J, Ogawa T, Tsuji E. Hepatotoxicity induced by trastuzumab used for breast cancer adjuvant therapy: a case report. J Med Case Rep. 2014;8:417. doi: 10.1186/1752-1947-8-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujii Y, Doi M, Tsukiyama N, Hattori Y, Ohya K, Shiroma N, Morio K, Morioka T, Aikata H, Shinozaki K, Chayama K. Sinusoidal obstruction syndrome post-treatment with trastuzumab emtansine (T-DM1) in advanced breast cancer. Int Cancer Conf J. 2020;9:18–23. doi: 10.1007/s13691-019-00392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe J, Ito Y, Saeki T, Masuda N, Takano T, Takao S, Nakagami K, Tsugawa K, Nakagawa S, Kanatani K, Nakayama T. Safety evaluation of trastuzumab emtansine in Japanese patients with HER2-positive advanced breast cancer. In Vivo. 2017;31:493–500. doi: 10.21873/invivo.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobert AM, Helms C, Larck C, Moore DC. Risk of hepatotoxicity with trastuzumab emtansine in breast cancer patients: a systematic review and meta-analysis. Ther Adv Drug Saf. 2020;11:2042098620915058. doi: 10.1177/2042098620915058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, Kubo T, Ninomiya T, Chikamori K, Harada D, Nogami N, Hirata T, Hinotsu S, Toyooka S, Kiura K. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol. 2018;13:273–279. doi: 10.1016/j.jtho.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 51.Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res. 2018;211:19–44. doi: 10.1007/978-3-319-91442-8_2. [DOI] [PubMed] [Google Scholar]
- 52.Dai C, Ma S, Wang F, Zhao H, Wu X, Huang Z, Chen Z, To K, Fu L. Lapatinib promotes the incidence of hepatotoxicity by increasing chemotherapeutic agent accumulation in hepatocytes. Oncotarget. 2015;6:17738–52. doi: 10.18632/oncotarget.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spraggs CF, Budde LR, Briley LP, Bing N, Cox CJ, King KS, Whittaker JC, Mooser VE, Preston AJ, Stein SH, Cardon LR. HLA-DQA1*02:01 is a major risk factor for lapatinib-induced hepatotoxicity in women with advanced breast cancer. J. Clin. Oncol. 2011;29:667–73. doi: 10.1200/JCO.2010.31.3197. [DOI] [PubMed] [Google Scholar]
- 54.Tangamornsuksan W, Chanprasert S, Nadee P, Rungruang S, Meesilsat N, Ueta M, Lohitnavy M. HLA-DRB1*07:01 and lapatinib-induced hepatotoxicity: a systematic review and meta-analysis. Pharmacogenomics J. 2020;20:47–56. doi: 10.1038/s41397-019-0092-2. [DOI] [PubMed] [Google Scholar]
- 55.Peroukides S, Makatsoris T, Koutras A, Tsamandas A, Onyenadum A, Labropoulou-Karatza C, Kalofonos H. Lapatinib-induced hepatitis: a case report. World J Gastroenterol. 2011;17:2349–52. doi: 10.3748/wjg.v17.i18.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012;2:a006577. doi: 10.1101/cshperspect.a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–9. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 58.Fairfax BP, Pratap S, Roberts IS, Collier J, Kaplan R, Meade AM, Ritchie AW, Eisen T, Macaulay VM, Protheroe A. Fatal case of sorafenib-associated idiosyncratic hepatotoxicity in the adjuvant treatment of a patient with renal cell carcinoma. BMC Cancer. 2012;12:590. doi: 10.1186/1471-2407-12-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klempner SJ, Choueiri TK, Yee E, Doyle LA, Schuppan D, Atkins MB. Severe pazopanib-induced hepatotoxicity: clinical and histologic course in two patients. J. Clin. Oncol. 2012;30:e264–8. doi: 10.1200/JCO.2011.41.0332. [DOI] [PubMed] [Google Scholar]
- 60.Spengler EK, Kleiner DE, Fontana RJ. Vemurafenib-induced granulomatous hepatitis. Hepatology. 2017;65:745–748. doi: 10.1002/hep.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haq MI, Nixon J, Stanley AJ. Imatinib and liver toxicity. BMJ Case Rep. 2018;11:e226740. doi: 10.1136/bcr-2018-226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda M, Okamoto I, Fukuoka M, Nakagawa K. Successful treatment with erlotinib after gefitinib-related severe hepatotoxicity. J. Clin. Oncol. 2010;28:e273–4. doi: 10.1200/JCO.2009.26.5496. [DOI] [PubMed] [Google Scholar]
- 63.Ku GY, Chopra A, Lopes Gde L Jr. Successful treatment of two lung cancer patients with erlotinib following gefitinib-induced hepatotoxicity. Lung Cancer. 2010;70:223–5. doi: 10.1016/j.lungcan.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Toba H, Sakiyama S, Takizawa H, Tangoku A. Safe and successful treatment with afatinib in three postoperative non-small cell lung cancer patients with recurrences following gefitinib/erlotinib-induced hepatotoxicity. J Med Invest. 2016;63:149–51. doi: 10.2152/jmi.63.149. [DOI] [PubMed] [Google Scholar]
- 65.Ueda H, Hayashi H, Kudo K, Takeda M, Nakagawa K. Successful treatment with afatinib after gefitinib- and erlotinib-induced hepatotoxicity. Invest New Drugs. 2016;34:797–799. doi: 10.1007/s10637-016-0384-1. [DOI] [PubMed] [Google Scholar]
- 66.Westgeest HM, van Erp NP, Honeywell RJ, Hoekstra R, Peters GJ, Verheul HM. Successful treatment of renal cell carcinoma with sorafenib after effective but hepatotoxic sunitinib exposure. J. Clin. Oncol. 2013;31:e83–6. doi: 10.1200/JCO.2012.43.6485. [DOI] [PubMed] [Google Scholar]
- 67.Harbaum L, Marx A, Goekkurt E, Schafhausen P, Atanackovic D. Treatment with dasatinib for chronic myeloid leukemia following imatinib-induced hepatotoxicity. Int J Hematol. 2014;99:91–4. doi: 10.1007/s12185-013-1474-x. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Wu Y, Dong M, He X, Wang Z, Li J, Wang Y. Observation of hepatotoxicity during long-term gefitinib administration in patients with non-small-cell lung cancer. Anticancer Drugs. 2016;27:245–50. doi: 10.1097/CAD.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao B, Zhao H. Incidence and risk of regorafenib-induced hepatotoxicity. Oncotarget. 2017;8:84102–84111. doi: 10.18632/oncotarget.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uetake H, Sugihara K, Muro K, Sunaya T, Horiuchi-Yamamoto Y, Takikawa H. Clinical features of regorafenib-induced liver injury in Japanese patients from postmarketing experience. Clin Colorectal Cancer. 2018;17:e49–e58. doi: 10.1016/j.clcc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Shantakumar S, Nordstrom BL, Djousse L, Hall SA, Gagnon DR, Fraeman KH, van Herk-Sukel M, Chagin K, Nelson J. Occurrence of hepatotoxicity with pazopanib and other anti-VEGF treatments for renal cell carcinoma: an observational study utilizing a distributed database network. Cancer Chemother Pharmacol. 2016;78:559–66. doi: 10.1007/s00280-016-3112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma Y, Xin S, Huang M, Yang Y, Zhu C, Zhao H, Zhang Y, Chen L, Zhao Y, Li J, Zhuang W, Zhu X, Zhang L, Wang X. Determinants of Gefitinib toxicity in advanced non-small cell lung cancer (NSCLC): a pharmacogenomic study of metabolic enzymes and transporters. Pharmacogenomics J. 2017;17:325–330. doi: 10.1038/tpj.2016.31. [DOI] [PubMed] [Google Scholar]
- 73.Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol. 2018;31:965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 74.Stucci S, Palmirotta R, Passarelli A, Silvestris E, Argentiero A, Lanotte L, Acquafredda S, Todisco A, Silvestris F. Immune-related adverse events during anticancer immunotherapy: pathogenesis and management. Oncol Lett. 2017;14:5671–5680. doi: 10.3892/ol.2017.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lleo A, Rimassa L, Colombo M. Hepatotoxicity of immune check point inhibitors: approach and management. Dig Liver Dis. 2019;51:1074–1078. doi: 10.1016/j.dld.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 76.Ahmed T, Pandey R, Shah B, Black J. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ Case Rep. 2015;2015:bcr2014208102. doi: 10.1136/bcr-2014-208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imafuku K, Yoshino K, Yamaguchi K, Tsuboi S, Ohara K, Hata H. Successful treatment of sudden hepatitis induced by long-term nivolumab administration. Case Rep Oncol. 2017;10:368–371. doi: 10.1159/000471480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurokawa K, Hara M, Iwakami SI, Genda T, Iwakami N, Miyashita Y, Fujioka M, Sasaki S, Takahashi K. Cholestatic liver injury induced by pembrolizumab in a patient with lung adenocarcinoma. Intern Med. 2019;58:3283–3287. doi: 10.2169/internalmedicine.2591-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anugwom C, Leventhal T. Nivolumab-induced autoimmune-like cholestatic hepatitis in a liver transplant recipient. ACG Case Rep J. 2020;7:e00416. doi: 10.14309/crj.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo X, Li W, Hu J, Zhu EC, Su Q. Hepatotoxicity in patients with solid tumors treated with PD-1/PD-L1 inhibitors alone, PD-1/PD-L1 inhibitors plus chemotherapy, or chemotherapy alone: systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:1345–1354. doi: 10.1007/s00228-020-02903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sermer D, Brentjens R. CAR T-cell therapy: full speed ahead. Hematol Oncol. 2019;37(Suppl 1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 82.Martinez M, Moon EK. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strati P, Nastoupil LJ, Fayad LE, Samaniego F, Adkins S, Neelapu SS. Safety of CAR T-cell therapy in patients with B-cell lymphoma and chronic hepatitis B or C virus infection. Blood. 2019;133:2800–2802. doi: 10.1182/blood.2019000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, Cheng H, Cao J, Shi M, Yan Z, Qiao J, Jing G, Wang X, Sang W, Xia R, Zhang X, Li Z, Gale RP, Zheng J, Zhu F, Xu K. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia. 2020;34:2704–2707. doi: 10.1038/s41375-020-0936-4. [DOI] [PubMed] [Google Scholar]
- 87.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, Gratama JW. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471–1482. doi: 10.1634/theoncologist.2015-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 90.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368:1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 91.Teo YL, Ho HK, Chan A. Formation of reactive metabolites and management of tyrosine kinase inhibitor-induced hepatotoxicity: a literature review. Expert Opin Drug Metab Toxicol. 2015;11:231–42. doi: 10.1517/17425255.2015.983075. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Cai Y, Zhang SR, Li CY, Jiang LL, Wei P, He MF. Mechanism of hepatotoxicity of first-line tyrosine kinase inhibitors: gefitinib and afatinib. Toxicol Lett. 2021;343:1–10. doi: 10.1016/j.toxlet.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018;38:976–987. doi: 10.1111/liv.13746. [DOI] [PubMed] [Google Scholar]


