Fig. 4. Comparison of conformational states of β-subunits.
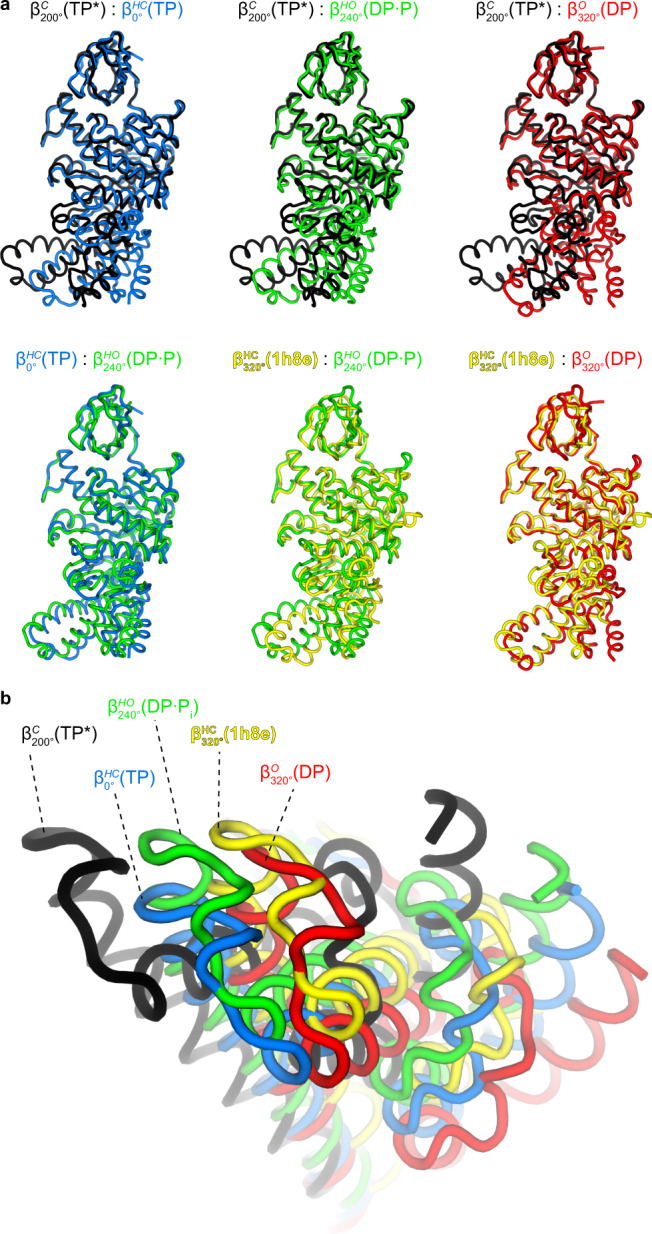
Superposition (on the N-terminal β-barrel) of the four main conformational states seen in the TF1(βE190D) (this study; , , , and ), and the half-open state observed for bMF120 (pdb1h8e). (black) is in a fully closed state, (red) is in a fully open state, and are intermediate structures that are either half closing or half opening, and is an intermediate structure of bMF1 similar to the open conformation. a Individual unique conformations compared to one other and viewed from the side. b Unique conformations superimposed and viewed from below.
