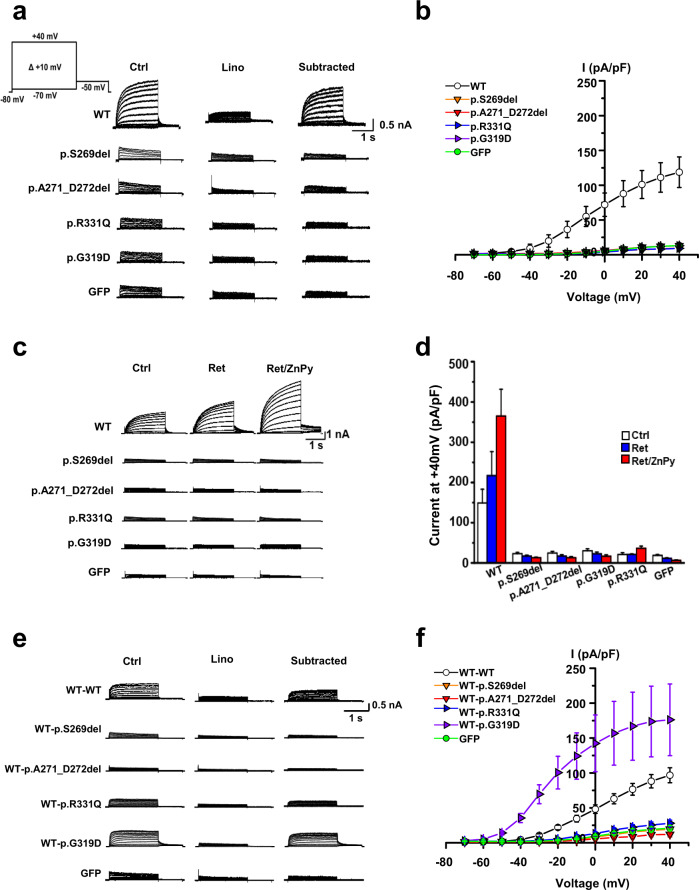
Fig. 2. Impaired channel conductance and dominant-negative effects of KCNQ4 mutant channels.
a Whole-cell K+ currents recorded from HEK293T cells transiently expressing KCNQ4 WT, p.S269del, p.A271_D272del, p.G319D, p.R331Q, or GFP. Linopirdine (30 μM)-sensitive K+ currents (‘subtracted’) from homomeric KCNQ4 mutant channels were barely detectable. b Current-voltage (I-V) relationships of linopirdine-sensitive K+ currents. c KCNQ openers did not activate homomeric KCNQ4 mutant channels. Retigabine (Ret, 10 μM) or a combination of Ret and zinc pyrithione (Ret/ZnPy 10 μM) did not activate mutant channels. d After treatment of KCNQ openers, the current densities of mutant channels were not significantly different from that of GFP (n = 7–9). e Representative K+ current traces recorded from KCNQ4 channels assembled from WT-WT, WT-p.S269del, WT-p.A271_D272del, WT-p.G319D, and WT-p.R331Q tandem concatemers. GFP-transfected cells were used as negative controls, and linopirdine-sensitive currents were subtracted for comparison. f I-V relationships of linopirdine-sensitive K+ currents of the concatemer channels (n = 4–21). Mean ± SEM.