Figure 1.
Assessment of exosomes produced by macrophages cultured in a high-glucose environment
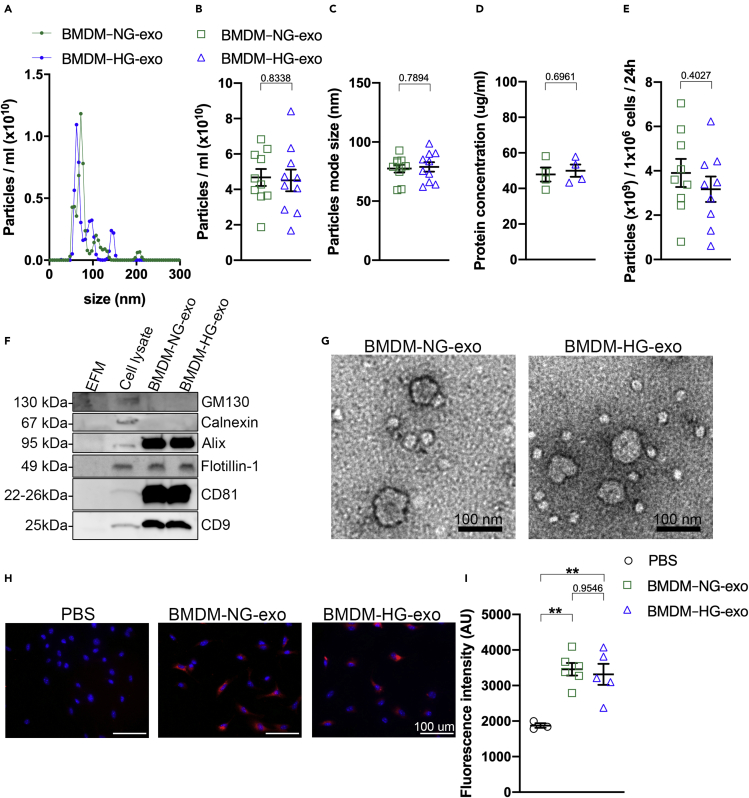
(A) Representative size and concentration distribution of BMDM–NG-exo or BMDM–HG-exo purified from BMDM conditioned cell culture supernatants after a 24 h period of culture. Measurement of particle concentration (B) and particle mode size (C) using nanoparticle tracking analysis.
(D) Quantification of protein concentration by qubit assay.
(E) Calculation of secreted particles amounts per million of BMDM in high glucose or low glucose conditions.
(F) Western blot analysis of GM130, Calnexin, Alix, Flotillin, and CD81 and CD9 in exosome-free media (EFM), cell lysate, and BMDM–derived exosomes (representative of three independent experiments). An equal volume (37.5 μL) of cDGUC fraction samples was loaded for exosomes analysis and 10 ug of protein was loaded for the cell lysate sample.
(G) Electron micrograph of purified exosomes from BMDM cells. Scale bars: 100 nm.
(H) Merged images showing internalization of PKH26–labeled BMDM exosomes (red) by naive culture BMDM counterstained with DAPI (blue). BMDM were co-incubated with 2x109 PKH26-labeled exosomes for 2 h at 37°C and washed repeatedly to remove unbound exosomes. All images were acquired using a using a Nikon microscope system with 20× objectives. Scale bars: 100μm.
(I) Quantification of the fluorescence intensity.∗p< .05; ∗∗p< .01 as determined by unpaired Student's t test analysis. Data are represented as mean ± SEM.