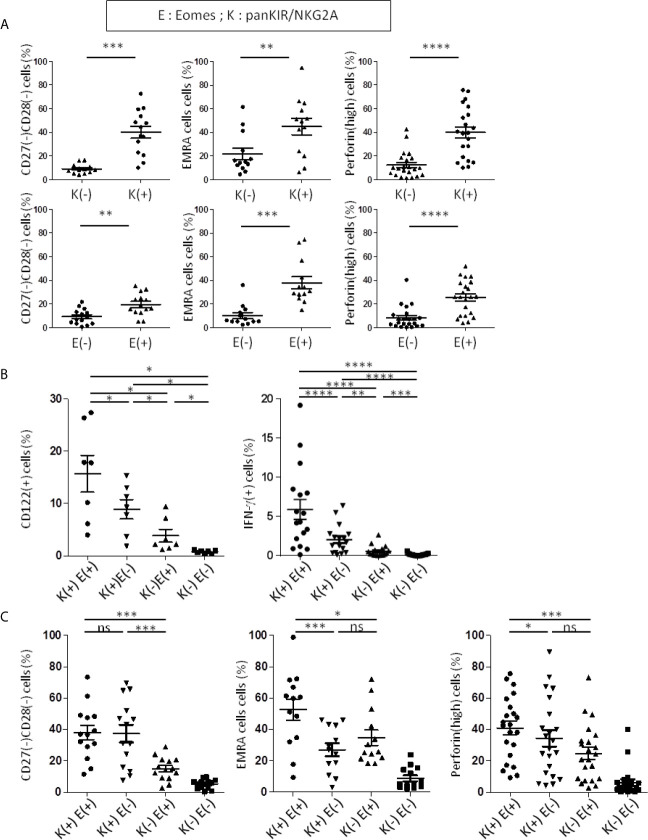
Figure 4.
PanKIR/NKG2A(+) Eomes(+) and panKIR/NKG2A(+) Eomes(-) CD8 T-cells share innate functions and a senescent/inflammaging-like signature. Flow cytometry analysis of TCRαβ(+) CD8(+) circulating live lymphocytes from healthy donors. (A) CD8 T-cells expressing panKIR/NKG2A or Eomes markers preferentially harbor a senescent signature and a terminal effector phenotype. The frequencies of senescent (CD27(-) CD28(-)) (n=14), EMRA (CD45RA(+) CCR7(-)) (n=13) and perforin(high)-expressing cells (n=22) among CD8 T-cells expressing panKIR/NKG2A (upper panel) or Eomes (lower panel) were analyzed. (B) panKIR/NKG2A and Eomes markers define two innate CD8 T-cell subsets besides their conventional memory and naive counterparts: evidence for an innateness gradient. CD122-expressing cells (n=7) ex vivo (left panel) and IFN-γ-producing cells (n=17) (right panel) frequencies after 48h stimulation with IL-12/IL-18 among the four CD8 T-cell subpopulations defined by panKIR/NKG2A and Eomes markers. Representative plots are shown in Figure S2 . (C) Innate CD8 T-cell subsets are enriched in senescent/terminal effector cells. Frequencies of senescent (CD27(-)CD28(-)) cells (n=14) (left panel), EMRA cells (n=13) (middle panel) and perforin(high)-expressing cells (n=22) (right panel) among the four CD8 T-cell subpopulations defined by panKIR/NKG2A and Eomes markers. Differences between E(-)K(-) and the other three cell populations, although significant, are not shown. Data are presented as mean ± SEM. Two-tailed Wilcoxon non-parametric test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, not significant. For detailed gating strategy, see Figure S1 .