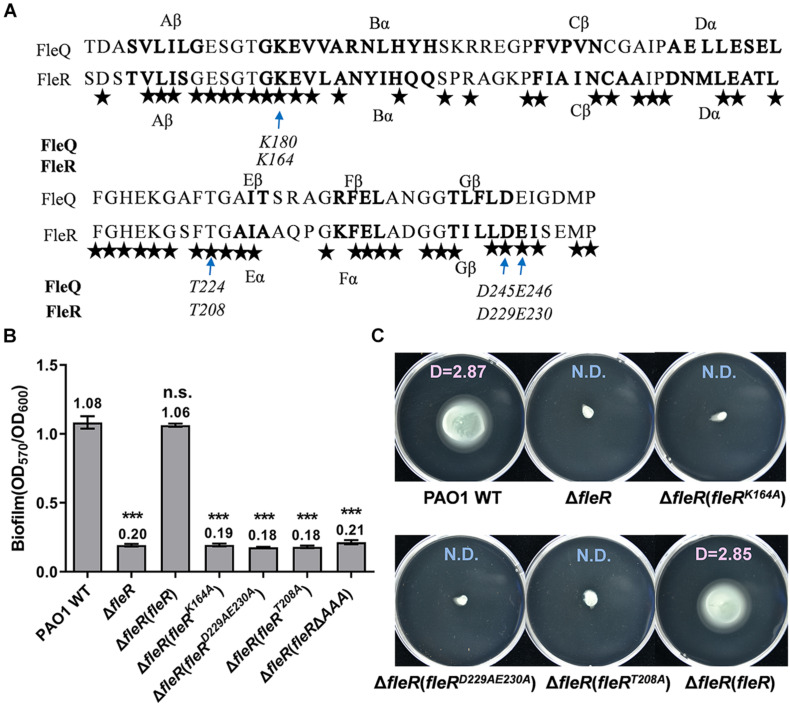
FIGURE 5.
Analysis of the conserved amino acids in the AAA domain of FleR. (A). The AAA domain of FleR displays a similar structural fold and shares the conserved key residues with the AAA domain of FleQ (WP_003086448.1). The α helix and β sheet of FleR and FleQ shown in bold were predicted using PredictProtein (http://cubic.bioc.columbia.edu/). The arrows indicate the conserved amino acid residues which are the key residues in the Walker A, Walker B and σ54-binding motifs in FleQ. All the identical residues in FleR and FleQ are indicated by stars. (B,C) The role of K164, D229&E230, T208 in FleR-AAA in controlling biofilm formation (B) and swimming motility (C) was examined by ectopic expression of FleR containing K164A, D229A&E230A, T208A in the ΔfleR strain, respectively. ***P < 0.001, n.s., no significance (Student’s t-test). Representative images and the average diameter (D) of the swimming migration zone of each strain are shown. N.D., not detected. Each experiment was performed in triplicate.