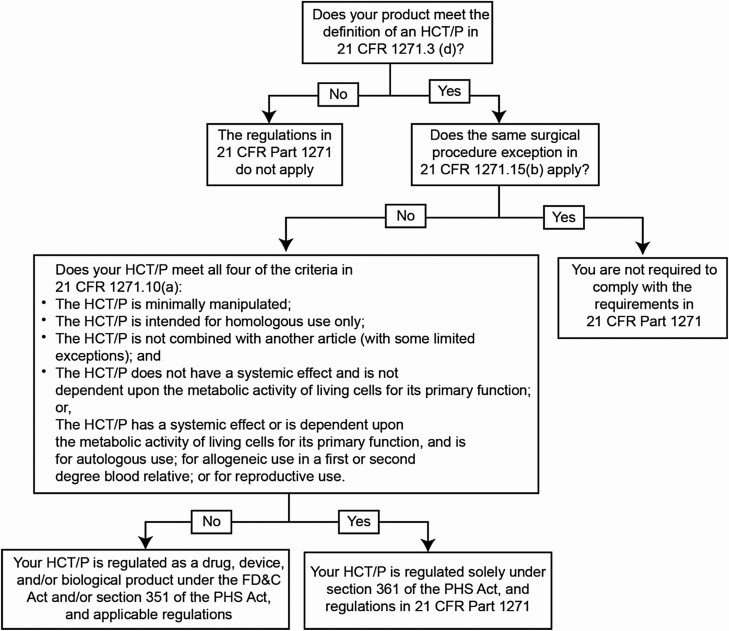
Figure 1.
Flowchart to illustrate how to apply the criteria in 21 CFR 1271.15(b) and 1271.10(a) for HCT/Ps. 21 CFR, Code of Federal Regulations Title 21; FD&C, Food, Drug, & Cosmetic Act; HCT/P, human cells, tissues, and cellular and tissue-based product; PHS, Public Health Service.8