FIGURE 2.
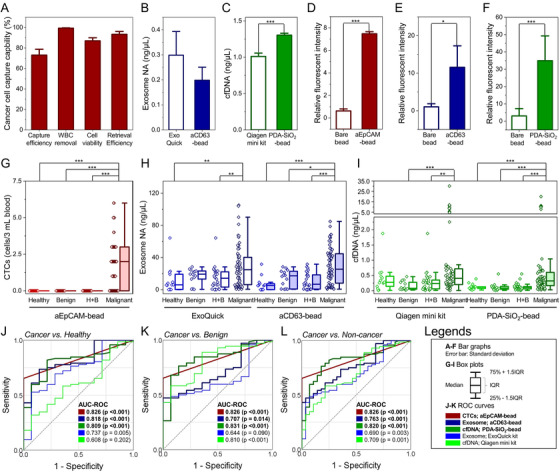
The diagnostic capability of the new bead‐based system for (A) CTCs, (B) exosomes, and (C) cfDNA tested using the human colorectal cancer cell line, SW480 cells. (D‐F) Target specificity of the bead‐based system, validated by comparing CTCs, exosomes, and cfDNA captured on each type of functionalized beads to those captured on bare alginate beads. Note that the capture of all three biomarkers was not prominent on the bare alginate beads, implying that the alginate itself does not contribute to the capture of tumor biomarkers. (G) Number of CTCs, (H) amount of exosome NA, and (I) concentration of plasma cfDNA quantified from a cohort consisting of 72 patients with malignant tumors, 14 patients with benign tumors, and 14 healthy individuals. For exosomes and cfDNA, the bead‐based system was compared with commercially available kits, ExoQuick kit, and Qiagen mini kit, respectively. (J‐L) ROC curves demonstrating the diagnostic capability of the new bead‐based system for distinguishing the patients with malignant tumors from healthy individuals, patients with benign tumors, and overall non‐cancer cohorts (healthy individuals + patients with benign tumors)
