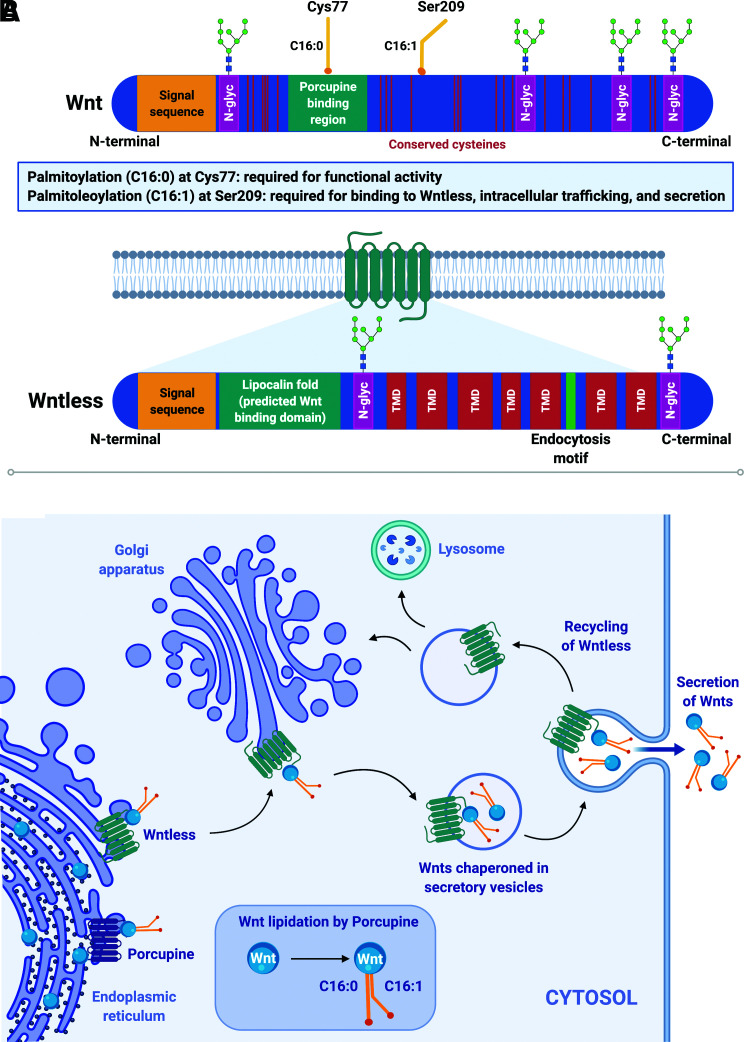
Figure 2.
Wnts play fundamental and diverse roles in tissue development and maintenance. A: Wnts are lipid-modified secreted glycoproteins (∼30–40 kDa) characterized by a signal peptide sequence, 23 conserved cysteine residues, varying numbers of N-glycosylation sites (N-glyc), and two conserved lipid modifications: palmitic acid (C16:0) at Cys77 and palmitoleic acid (C16:1) at Ser209. Palmitoleoylation (C16:1) at Ser209 is required for the interaction between Wnts and their dedicated chaperone protein, Wntless; palmitoylation (C16:0) at Cys77 is required for functionality of secreted Wnts. Wntless is an evolutionarily conserved transmembrane protein required for intracellular trafficking and secretion of lipidated Wnts. Wntless (∼62 kDa) is predicted to have seven transmembrane domains (TMD), a signal peptide sequence, an endocytosis motif, and a hydrophobic lipocalin domain thought to be the site of interaction with Wnts. B: After Wnt proteins are synthesized in the ER, they undergo significant posttranslational modifications, including N-glycosylation and lipidation. The ER acyltransferase Porcupine catalyzes addition of palmitic (C16:0) and palmitoleic (C16:1) acid moieties at conserved Cys77 and Ser209 residues, respectively. Wntless binds to and chaperones Wnt proteins from the ER through the trans-Golgi network and to the plasma membrane in secretory vesicles. Once Wnts are secreted, Wntless is transported back via a retromer complex to the Golgi for reuse or to lysosomes for degradation.