Summary
There is large heterogeneity in approaches to tackling nosocomial outbreaks caused by carbapenemase-producing Enterobacterales (CPE), however there is limited guidance on how to approach their management. Rapid and robust infection prevention and control interventions can be effective in preventing and reducing the impact of outbreaks in healthcare environments. We present a stepwise approach to aspects of CPE outbreak management, including the development of an action plan, engagement and communication with key stakeholders, developing a dynamic risk assessment, and staff education. These can provide a blueprint for organisations to create templates and checklists to inform their own outbreak response.
Introduction
Carbapenem antibiotics are widely used for treating Gram-negative infections that have developed resistance to other, previously effective, classes of antibiotics. The emergence of resistance to these ‘last resort’ antibiotics poses a threat to the provision of healthcare to individual patients and to the wider system. Reports of outbreaks of colonisation or infection with carbapenemase-producing Enterobacterales (CPE) are increasing globally and in the UK. Many are probably missed, particularly where testing for resistance mechanisms is not routine; additionally plasmids carrying resistance mechanisms can easily spread between genera making outbreaks more challenging to identify [1]. Given the associated morbidity and mortality, health systems must take robust steps to prevent, detect and control CPE outbreaks [2]. Endemicity within healthcare settings is the likely consequence of a failure to curtail CPE spread, with associated disruption to services and impact on patients.
Conventionally an outbreak is described as ‘a similar illness or infection in two or more people linked in time and space’. However, a single occurrence of CPE (in a colonised or infected patient) may indicate an unrecognised outbreak; if the patient was not identified as high risk on admission, transmission may have already occurred in the absence of infection prevention and control (IPC) interventions [3,4].
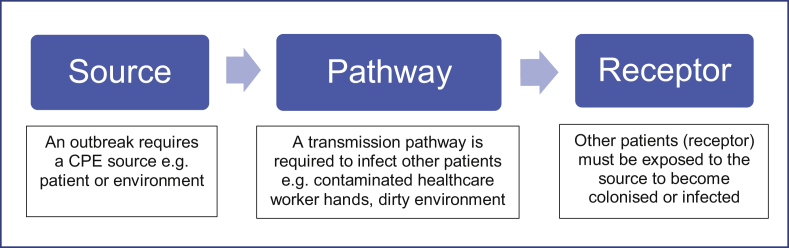
While the evidence base for the prevention and control of outbreaks of CPE is still emerging, when robust infection prevention and control interventions are applied, transmission can be controlled. Fundamentally, the steps to prevent or interrupt outbreaks are simple, however the implementation is difficult but necessary as these organisms are unforgiving of any lapse in IPC. The steps to prevent or interrupt transmission and therefore prevent outbreaks can be summarised through the “source > pathway > receptor” model, as seen in Figure 1.
Figure 1.
Transmission model.
Providers should consider the steps of care pathways with this model to determine where transmission events may occur and take steps to mitigate them. Using the logic that separation of any of these three components breaks the chain of transmission, the following recommendations have been developed to prevent and control clusters or outbreaks of CPE in healthcare settings. Full guidance on the management of CPE in health and social settings can be accessed at https://www.gov.uk/health-and-social-care/antimicrobial-resistance.
These recommendations were developed through expert consensus by a working group convened to inform the production of national guidelines and were informed by clinical and public health experience and published scientific literature.
Key aspects of CPE outbreak control
Robust planning, effective leadership, collaboration and communications (including the provision of clear explanations and information for patients) are key to the control of CPE. We detail key recommendations in subsequent sections.
Engage senior management
Engaging organisational and local health and social care senior management is vital; senior management should make it their business to be involved as they are best-placed to champion IPC work and retain ownership of the delivery of health services [5]. A recent review of the implementation of the English guidance ‘Acute trust toolkit for the early detection, management and control of carbapenemase-producing Enterobacteriaceae’ (hereafter ‘CPE toolkit’), noted that senior management engagement resulted in easier and better resourced response planning [6].
Develop a CPE management and outbreak plan
Healthcare providers should develop and regularly review and test (exercise) a local CPE outbreak management plan for responding to a general increase in cases, a local cluster or wider outbreak. Key components of the plan should include activities to increase engagement and understanding, as well as the optimisation of processes and practices; these are further set out in Box A.
Box A. Components of a CPE Management and Outbreak Plan.
-
•
Develop local arrangements for an outbreak control team, including involvement of senior management
-
•Conduct dynamic risk assessments that account for the evolution of outbreak scenarios:
-
-Review current epidemiology in the hospital and locality in IPC meetings, incident meetings and other relevant fora
-
-Ensure robust and rapid detection of colonised or infected cases through optimised laboratory methods [7] and surveillance, including the scrutiny of data relating to unusual isolates and trends
-
-Develop and implement isolation approaches for single cases and larger cluster/outbreak situations
-
-Optimise IPC practice and cleaning approaches, including audits
-
-Monitor and manage antibiotic pressures through embedded antimicrobial stewardship programmes
-
-Determine and implement staffing requirements to manage an outbreak situation
-
-Ensure approaches to internal and external patient transfers are optimised to minimise the risk of infection transmission
-
-
-
•
Develop an outbreak communications plan for internal and external communications, including information for patients
-
•
Identify, develop and test effective cascade methods to provide rapid reminders of the need for strict adherence to the ‘CPE Management and Outbreak Plan’ and standard IPC operating procedures to relevant staff
-
•
Develop education programmes for all staff to ensure that there is good understanding of CPE and that staff are clear of their role(s) in the management of an outbreak situation.
Alt-text: Box A
Local arrangements for an outbreak control team
An outbreak or incident control team (OCT) is required to risk assess, coordinate and manage the response to an outbreak, cluster or an increase in cases above the usual baseline.
OCT meetings should follow a standard, minuted agenda to ensure all aspects of outbreak control are considered and actioned. A dynamic risk assessment of the outbreak should be developed along with an action plan identifying roles and responsibilities (regularly revised as an outbreak evolves) to guide the required response and control measures.
Dynamic risk assessment and action plan
A dynamic risk assessment (adapted from Lepelletier [8]) and action plan should address the points detailed in Table 1.
Box B. Cohort groups.
The following discrete groups can be considered for cohorting [8,11]:
-
1.
Patients and healthcare staff for colonised or infected patients.
-
2.
Contacts of colonised or infected patients (i.e. contacts of colonised or infected patients and the staff that have looked after them).
-
3.
Newly admitted patients and healthcare staff.
-
4.
Screen negative patients and healthcare staff.
Alt-text: Box B
Table 1.
Aspects for inclusion and consideration when conducting a dynamic risk assessment to contain and control CPE in healthcare settings
| Component | Considerations |
|---|---|
| Type of patients |
|
| Identification of colonised and infected patients, including surveillance |
|
| Laboratories and IPC teams should implement systems for tracking patients with key infections |
|
| Staff-patient ratios and IPC expertise |
|
| IPC guidelines and standards |
|
| Environment |
|
| Isolation capacity on the ward or unit |
|
| Manage antibiotic pressures in the healthcare facility |
|
Other containment measures
Restriction of colonised or infected inpatient movements to other departments should be considered to reduce transmission risks. Known colonised or infected patients should be put at the end of the day's list of work for a department (e.g. X ray, other clinic appointment, operating list), providing it is clinically safe to do so and clinical outcomes are not compromised, so that terminal cleaning can be undertaken after the case has left that department.
Ward or unit closures may be required, to try to contain onward transmission (e.g. to allow for a systematic deep clean), however efforts to contain an outbreak or cluster should be balanced against the wider safety of patients as there may be the potential for greater harm if patients are delayed in receiving their treatments.
Closure of wards/units clearly has an impact on healthcare operational capacity and may impact on the availability of scarce facilities e.g. specialised units; nonetheless closure can be a valuable intervention tool. Providers need to have thought through and developed standard operating procedures prior to CPE incidents that describe the situations when closure should be considered, the steps to be taken, including mitigation of risk to patients from loss of specialist services and the procedures for planned re-opening.
The risk of transmission from patients who are currently or previously CPE colonised attending outpatient clinics is unclear, but it is generally considered that provided they can exercise good hand hygiene and toileting practice and have any wounds covered, they should not need to be restricted in their movements. Some have adopted a precautionary approach and cohorted these patients [37]. Providers will need to make their own risk assessed judgement based on the local context.
Communication
Effective communication is key when transferring patients colonised or infected with CPE to other units or wards in the healthcare facility, or to other sites (e.g. care homes or other hospitals).
Relevant information should be cascaded to all cadres if healthcare and allied staff who need to be aware of the scale of the outbreak, including any remedial measures or changes to routine practice they should be aware of. Managerial oversight of the incident response needs to be owned by a dedicated individual with regular reports to the Trust board and senior infection staff.
Outbreaks should be communicated to relevant bodies such as neighbouring trusts, commissioners, providers and the local PHE Health Protection Team. The sending unit should outline the nature of the colonisation or infection of the patient and the containment measures required to the receiving unit.
Individual patient-level information should be shared on discharge with all members of the patient's care team, such as their General Practitioner, district nursing team, other facilities involved in their care, and family and carers. Discharge information to the primary care provider should be clear, including treatment issues or difficulties with infection control in shared care environments such as care homes. These are new concepts to primary care and a detailed explanation may be required; an explanatory leaflet to primary care practitioners may help.
Describe and report outbreak features to stakeholders
A key step in an outbreak response is to describe the outbreak, which can inform hypothesis generation and associated investigations to identify the source of the outbreak. Resulting findings should inform the development and implementation of prevention and control measures. Case definitions for cases and contacts should be established and the outbreak data used to determine epidemiological links and potential sources including environmental reservoirs. A report summarising these findings should be produced, updated and shared regularly, including an outbreak curve, patient movement mapping, patient network analysis (who has been in contact with whom) and notes of procedures undertaken (e.g. surgery, vascular lines) to identify potential exposure risks.
An analytical study (case control or cohort study) may help to further identify causal risk factors for informing further control measures. It is important that the current epidemiological situation is reviewed in organisational IPC, OCT and healthcare facility management meetings regularly so that the incident management can be adjusted in the light of the current position.
Develop educational programmes for all staff
A review of the implementation of the ‘CPE toolkit’ noted front line healthcare staff (i.e. not IPC experts) had little knowledge or understanding of the threat posed by CPE [6]. Improving staff knowledge is therefore an important element of outbreak preparation and may improve their involvement and compliance with the required IPC activities. Healthcare providers should ensure that education on IPC and staff roles in an outbreak response is provided in advance of and during an outbreak. Appropriate multimodal approaches, according to staff needs, should focus on educational outcomes and increasing awareness. The approach to educational programmes should acknowledge the difficulties operational pressures may pose to staff engagement [9,38]. Refresher training is important to maintain knowledge, and raising awareness is particularly important during outbreak situations.
Educational programmes should encompass as a minimum:
-
•
Context and role-appropriate knowledge of IPC management (all staff)
-
•
Context and role-appropriate basic microbiology (all staff)
-
•
Current policy for patient screening, including how to take a screening swab and refer to microbiology services for testing (all staff)
-
•
Current best practices for prescribing, administering and monitoring antimicrobial therapy (staff prescribing and administering medications to patients)
-
•
Importance of antimicrobial stewardship; careful conservation of existing antimicrobials (staff prescribing and administering medications to patients)
-
•
Effect on individual patients' mental and emotional health whilst in isolation and how to communicate effectively when giving patients factual information in a format that they will understand
-
•
Awareness of current outbreak procedures and the response required of staff
Conclusions
CPE outbreaks have the potential to cause widespread disruption to clinical services, limit the ability to perform medical procedures, cause harm and emotional stress to individual patients, and have widespread financial and reputational consequences. Adequate surveillance must be maintained to detect, at an early stage, introduction of CPE into the hospital environment to contain any transmission. An advance plan is essential to ensure: that appropriate admission samples are taken from high-risk patients and from patients on high-risk units (e.g. burns, transplant units); that CPE isolates can be adequately detected by local laboratories; that information is regularly reviewed to determine if transmission has occurred, and; that outbreak management strategies are in place, appropriately stress tested and regularly updated, with education provided to key staff. Ultimately, local risk assessments looking at the type of facility (including isolation capacity – especially on high risk units), patients, and available control measures are key to ensuring that risk is appropriately identified and mitigated. Addressing the points contained in this paper we hope will help institutions in developing their own robust local strategies to combat the spread of CPE.
Conflict of interest statement
None declared.
References
- 1.van Duin D., Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8(4):460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budhram D.R., Mac S., Bielecki J.M., Patel S.N., Sander B. Health outcomes attributable to carbapenemase-producing Enterobacteriaceae infections: A systematic review and meta-analysis. Infect Contr Hosp Epidemiol. 2020;41(1):37–43. doi: 10.1017/ice.2019.282. [DOI] [PubMed] [Google Scholar]
- 3.Institut National de Sante Publique du Quebec . 2016. Measures to prevent and control transmission of multidrug-resistant Gram negative bacilli in acute care settings in quebec. [Google Scholar]
- 4.HSC Public Health Agency. The Northern Ireland regional infection prevention and control manual Available from: https://www.niinfectioncontrolmanual.net/outbreak-management.
- 5.Zingg W., Holmes A., Dettenkofer M., Goetting T., Secci F., Clack L. Hospital organisation, management, and structure for prevention of health-care-associated infection: a systematic review and expert consensus. Lancet Infect Diseases. 2015;15(2):212–224. doi: 10.1016/S1473-3099(14)70854-0. [DOI] [PubMed] [Google Scholar]
- 6.Schneider A., Coope C., Michie S., Puleston R., Hopkins S., Oliver I. Implementing a toolkit for the prevention, management and control of carbapenemase-producing Enterobacteriaceae in English acute hospitals trusts: a qualitative evaluation. BMC Health Serv Res. 2019;19(1):689. doi: 10.1186/s12913-019-4492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.England P.H. 2019. Commercial assays for the detection of acquired carbapenemases. [Google Scholar]
- 8.Lepelletier D., Berthelot P., Lucet J.C., Fournier S., Jarlier V., Grandbastien B. French recommendations for the prevention of ‘emerging extensively drug-resistant bacteria’ (eXDR) cross-transmission. J Hosp Infect. 2015;90(3):186–195. doi: 10.1016/j.jhin.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . 2016. Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level. [PubMed] [Google Scholar]
- 10.Thurlow C.J., Prabaker K., Lin M.Y., Lolans K., Weinstein R.A., Hayden M.K. Anatomic Sites of Patient Colonization and Environmental Contamination with Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae at Long-Term Acute Care Hospitals. Infect Control Hosp Epidemiol. 2013:56–61. doi: 10.1086/668783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepelletier D., Lucet J.C., Astagneau P., Coignard B., Vaux S., Rabaud C. Control of emerging extensively drug-resistant organisms (eXDRO) in France: a survey among infection preventionists from 286 healthcare facilities. Eur J Clin Microbiol Infect Diseases. 2015;34(8):1615–1620. doi: 10.1007/s10096-015-2396-8. [DOI] [PubMed] [Google Scholar]
- 12.Clarivet B., Pantel A., Morvan M., Jean Pierre H., Parer S., Jumas-Bilak E. Carbapenemase-producing Enterobacteriaceae: use of a dynamic registry of cases and contacts for outbreak management. J Hosp Infect. 2016;92(1):73–77. doi: 10.1016/j.jhin.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Tacconelli E., Mazzaferri F., de Smet A.M., Bragantini D., Eggimann P., Huttner B.D. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25(7):807–817. doi: 10.1016/j.cmi.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Giufrè M., Ricchizzi E., Accogli M., Barbanti F., Monaco M., Pimentel de Araujo F. Colonization by multidrug-resistant organisms in long-term care facilities in Italy: a point-prevalence study. Clinical Microbiology and Infection. 2017;23(12):961–967. doi: 10.1016/j.cmi.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Latour K., Huang T.-D., Jans B., Berhin C., Bogaerts P., Noel A. Prevalence of multidrug-resistant organisms in nursing homes in Belgium in 2015. PLOS ONE. 2019;14(3) doi: 10.1371/journal.pone.0214327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim C.J., Kong D.C.M., Cheng A.C., Spelman D., Peleg A.Y., Kennon J. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case–control study. J Antimicrobial Chemotherapy. 2014;69(7):1972–1980. doi: 10.1093/jac/dku077. [DOI] [PubMed] [Google Scholar]
- 17.Fagerström L., Kinnunen M., Saarela J. Nursing workload, patient safety incidents and mortality: an observational study from Finland. BMJ Open. 2018;8(4) doi: 10.1136/bmjopen-2017-016367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson J.J.N.T. The effects of nurse to patient ratios. Nurs Times. 2011;107(2):22–25. [PubMed] [Google Scholar]
- 19.Shekelle P.G. Nurse–Patient Ratios as a Patient Safety Strategy: A Systematic Review. Annals Int Med. 2013;158(5_Part_2):404–409. doi: 10.7326/0003-4819-158-5-201303051-00007. [DOI] [PubMed] [Google Scholar]
- 20.Teerawattanapong N., Kengkla K., Saokaew S., Dilokthornsakul P., Chaiyakunapruk N., Apisarnthanarak A. Prevention and Control of Multidrug-Resistant Gram-Negative Bacteria in Adult Intensive Care Units: A Systematic Review and Network Meta-analysis. Clin Infect Diseases. 2017;64(suppl_2):S51–S60. doi: 10.1093/cid/cix112. [DOI] [PubMed] [Google Scholar]
- 21.Carling P., Parry M.M., Rupp M., Po J., Dick B., Von Beheren S. Improving Cleaning of the Environment Surrounding Patients in 36 Acute Care Hospitals. Infect Control Hosp Epidemiol. 2008:1035–1041. doi: 10.1086/591940. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell B.G., Dancer S.J., Anderson M., Dehn E. Risk of organism acquisition from prior room occupants: a systematic review and meta-analysis. J Hosp Infect. 2015;91(3):211–217. doi: 10.1016/j.jhin.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Nseir S., Blazejewski C., Lubret R., Wallet F., Courcol R., Durocher A. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clinical Microbiology and Infection. 2011;17(8):1201–1208. doi: 10.1111/j.1469-0691.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramsburg A.M., Weingarten R.A., Conlan S.P., Dekker J.P., Michelin A.V., Odom R.T. Tracking an Unusual Carbapenemase-producing Organism from Drains to Patient Using Whole Genome Sequencing. Open Forum Infect Diseases. 2017;4(suppl_1):S33–S34. [Google Scholar]
- 25.Chemaly R.F., Simmons S., Dale C., Jr., Ghantoji S.S., Rodriguez M., Gubb J. The role of the healthcare environment in the spread of multidrug-resistant organisms: update on current best practices for containment. Ther Adv Infect Dis. 2014;2(3–4):79–90. doi: 10.1177/2049936114543287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado Naranjo J., Villate Navarro J.I., Sota Busselo M., Martínez Ruíz A., Hernández Hernández J.M., Torres Garmendia M.P. Control of a clonal outbreak of multidrug-resistant Acinetobacter baumannii in a hospital of the Basque country after the introduction of environmental cleaning led by the systematic sampling from environmental objects. Interdis Perspect Infect Diseases. 2013;2013 doi: 10.1155/2013/582831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferies J.M.C., Cooper T., Yam T., Clarke S.C. Pseudomonas aeruginosa outbreaks in the neonatal intensive care unit – a systematic review of risk factors and environmental sources. J Med Microbiol. 2012;61(8):1052–1061. doi: 10.1099/jmm.0.044818-0. [DOI] [PubMed] [Google Scholar]
- 28.Aranega-Bou P., George R.P., Verlander N.Q., Paton S., Bennett A., Moore G. Carbapenem-resistant Enterobacteriaceae dispersal from sinks is linked to drain position and drainage rates in a laboratory model system. J Hosp Infec. 2019;102(1):63–69. doi: 10.1016/j.jhin.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler D.L., Major Y., Bearman G., Edmond M.B. Transmission of nosocomial pathogens by white coats: an in-vitro model. J Hosp Infect. 2010;75(2):137–138. doi: 10.1016/j.jhin.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Lakdawala N., Pham J., Shah M., Holton J. Effectiveness of Low-Temperature Domestic Laundry on the Decontamination of Healthcare Workers' Uniforms. Infect Cont Hosp Epidemiol. 2011;32(11):1103–1108. doi: 10.1086/662183. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell A., Spencer M., Edmiston C. Role of healthcare apparel and other healthcare textiles in the transmission of pathogens: a review of the literature. J Hosp Infect. 2015;90(4):285–292. doi: 10.1016/j.jhin.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiener-Well Y., Galuty M., Rudensky B., Schlesinger Y., Attias D., Yinnon A.M. Nursing and physician attire as possible source of nosocomial infections. Am J Infect Cont. 2011;39(7):555–559. doi: 10.1016/j.ajic.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 33.De Geyter D., Blommaert L., Verbraeken N., Sevenois M., Huyghens L., Martini H. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control. 2017;6:24. doi: 10.1186/s13756-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leitner E., Zarfel G., Luxner J., Herzog K., Pekard-Amenitsch S., Hoenigl M. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother. 2015;59(1):714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martischang R., Buetti N., Balmelli C., Saam M., Widmer A., Harbarth S.J.A.R. Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrob Resist Infect Contr. 2019;8(1):37. doi: 10.1186/s13756-019-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonten M.J.M. Colonization pressure: a critical parameter in the epidemiology of antibiotic-resistant bacteria. Crit Care (London, England) 2012;16(4):142. doi: 10.1186/cc11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henderson D.K., Palmore T.N. Managing Transmission of Carbapenem-Resistant Enterobacteriaceae in Healthcare Settings: A View From the Trenches. Clin Infect Diseases. 2013;57(11):1593–1599. doi: 10.1093/cid/cit531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M.H., Lee G.A., Lee S.H., Park Y.H. Effectiveness and core components of infection prevention and control programmes in long-term care facilities: a systematic review. J Hosp Infect. 2019;102(4):377–393. doi: 10.1016/j.jhin.2019.02.008. [DOI] [PubMed] [Google Scholar]