Summary
Antimicrobial stewardship programs (ASP) are an essential practice to prevent increasing resistance against antibiotics. A successful ASP monitors not only prescribing patterns and practices but also contributes in minimizing the toxic effects of antibiotics. Moreover, ASP also facilitates the selection of disease specific antibiotics and enforces rules and regulations to rationalize the use of antibiotics. The aim of the study is to highlight the core elements of Hospital Antibiotic Stewardship Programs in Karachi. The key elements proposed by center of disease control (CDC) such as; leadership, accountability, drug expertise, actions to support optimal antibiotic use, tracking (monitoring antibiotic prescribing, use and resistance), reporting information to staff on improving antibiotic use and resistance and education were evaluated on Yes/No scale. The data was collected from 44 hospitals of different categories in Karachi and all the major elements were studied. It was observed that all the hospitals in one setting failed to comply with all the guidelines. It has been concluded that efforts should be made to design ASP at each hospital and implemented through suitable policies and procedures.
Keywords: Antibiotic, Hospital, Stewardship, CDC, Resistance
Introduction
In the recent years, resistance against antibiotics has developed due to extensive use of antibiotics in hospitals particularly against nosocomial infections [1]. It has been estimated that about 30–50% of the antibiotic consumption in hospitals is inappropriate [2]. The causative factors include; length of stay in hospital, mortality rate, antimicrobial daily doses and prevalence of multi drug resistance infections. The initiation of Antimicrobial stewardship programs (ASP) can markedly reduce antimicrobial utilization, without extending hospital stay and mortality [3,4].
Resistant bacteria, such as Acinetobacter baumannii carry serious treatment challenges and are on the rise worldwide. Cases of multi-drug and extensively-drug resistance Acinetobacter baumannii has increased, with pan-resistance strains emerging [5]. Social barriers have also been contributing factors to increased antimicrobial usage including; lack of awareness of resistance, unclear value of antibiotic clinical guidelines and hospital prescribing command [5].
Simultaneously, decline in the availability of new pharmaceutical agents increase challenges for physicians [6]. The effect of drug shortages have an impact on, for example, the shortage of piperacillin-tazobactam on meropenem consumption which have a subsequent effect on antimicrobial stewardship activity. ASP should closely monitor shortages and establish hands-on strategies to prevent improper consumption of antimicrobials [7].
ASP in hospitals aims to optimize antimicrobial prescribing to improve patient care, slow down the progression of antimicrobial resistance and to reduce hospital costs [8]. Guidelines from Infectious Diseases Society of America suggest that every hospital should develop a programme to improve prescribing. It also recommends various interventions to reduce improper antimicrobial utilization, to improve their rational selection, dosing, route and treatment duration and to minimize undesirable outcomes, such as adverse drug events, development of resistance, selection of pathogenic organisms and cost [4]. The appropriate use of antimicrobial agents has become an important aspect of patient safety with quality assurance that manages medication errors, allergy identification, and drug–drug interactions [9]. Commonly, ASP targeted patients in critical care units [10], an area of high antimicrobial use [11] and the epicenter of antimicrobial resistance in many hospitals [12]. ASP can be financially self-supporting through improve patient care [13].
The application of a ASP at facilities where inadequate infectious disease resources were present, could be practiced by pharmacists concentrating on basic interventions [14]. In this lower resource setting, acute care facilities were more likely to involve in ASP than critical care facilities, although critical care settings have the need for, development and improvements in this area [15].
Aims and objectives
The aim of this study is to identify the status of ASP in hospitals in Karachi. This survey will be helpful to for patient safety and build the future strategies of prescribing antibiotics.
Materials and method
An electronic questionnaire proposed by Centre of disease control to scientifically evaluate major aspects in rationalizing antibiotic prescribing was utilized. It contained 7 Core elements and 39 items. Core elements were; 1) Leadership 2) accountability 3) Drug expertise 4) actions to support optimal antibiotic use 5) Tracking: Monitoring antibiotic prescribing, use and resistance 6) Reporting information to staff on improving antibiotic use and resistance and 7) Education on Antimicrobial stewardship. These were measured on Yes or No scale [16].
As per economic survey of Pakistan 2016–2017 there are 1201 hospitals, 5518 Basic health Units, 683 Rural Health Centers, 5802 Dispensaries, 731 Maternity & Child Health Centers and 347 TB centers. The total availability of beds in these health facilities is estimated at 123394 in Pakistan [17].
Data was collected by the doctors, pharmacists or administrative persons using a convenience sampling method from the hospitals in Karachi, Pakistan. These enrolled hospitals were voluntarily willing to share their data, whilst maintaining confidentiality through a self-administrated questionnaire. Overall, more than 50 hospitals were approached for data collection which included government, non-government and corporate hospitals. Data was collected during the month of June and July 2018.
Results
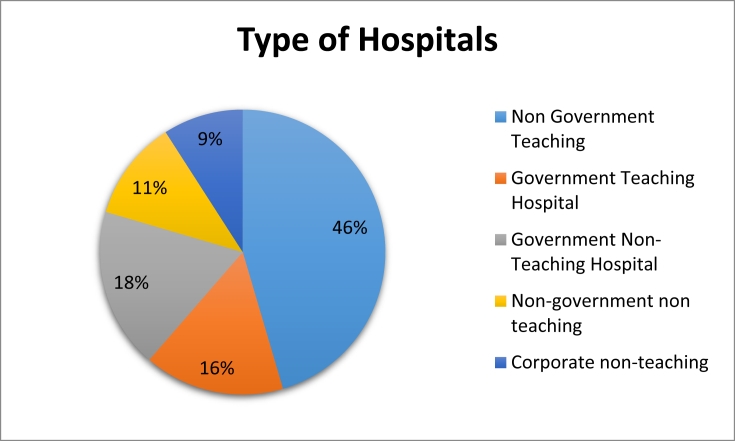
44 of the hospitals contacted submitted their data (Fig. 1). The overall statistics showed that only 34% hospitals provide a formal statement to support the ASP and only 27% hospitals receive financial support for antibiotic stewardship. As shown in Table 1, in 45% of hospitals a physician/pharmacist leader is responsible for program outcomes from stewardship activities, in 73% of hospitals a pharmacist leader responsible for improving antibiotic usage.
Fig. 1.
Type of hospitals.
Table 1.
Leadership and accountability.
| Leadership Support: Management commitment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Non government teaching |
Government teaching |
Government non-teaching |
Non-government non teaching |
Corporate non-teaching |
Overall |
||
| TR |
TR |
TR |
TR |
TR |
TR | Yes | No | |
| (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | ||||
| “Does your facility have a formal, written statement of support from leadership that supports efforts to improve antibiotic use (antibiotic stewardship)?” | 20 (75%) (25%) | 7 (0%) (100%) | 8 (0%) (100%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 34% | 66% |
| “Does your facility receive any budgeted financial support for antibiotic stewardship activities (e.g., support for salary, training, or IT support)?” | 20 (60%) (40%) | 7 (0%) (100%) | 8 (0%) (100%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 27% | 73% |
| Accountability: Accountability & responsibility | ||||||||
| “Is there a physician/Pharmacist leader responsible for program outcomes of stewardship activities at your facility?” | 20 (80%) (20%) | 7 (0%) (100%) | 8 (50%) (50%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 45% | 23% |
| “Is there a pharmacist leader responsible for working to improve antibiotic use at your facility?” | 20 (95%) (5%) | 7 (29%) (71%) | 8 (75%) (25%) | 5 (60%) (40%) | 4 (50%) (50%) | 44 | 73% | 23% |
Table 2 includes data on policies, procedures and procedural interventions to improve rationale use of antibiotic in different types of hospitals. Table 2 shows that 43% hospitals have a policy that require prescribers to document in the medical records a dose, duration, and indication for all antibiotic prescriptions. 36% of hospitals have facility-specific treatment recommendations, based on national guidelines and local susceptibility patterns. Overall, 55% hospitals have formal procedures for clinicians to review the appropriateness of all antibiotics 48 h after the initial order (example, antibiotic time out), 66% hospitals specified antibiotic agents needed to be approved by a physician or pharmacist prior to dispensing (i.e., pre-authorization) and 61% of hospitals had physician or pharmacist to review the courses of therapy for specified antibiotic agents (i.e. Prospective audit with feedback) as shown in Table 2.
Table 2.
Action.
| Items | Non government teaching |
Government teaching |
Government non-teaching |
Non-government non teaching |
Corporate non-teaching |
Overall |
||
|---|---|---|---|---|---|---|---|---|
| TR |
TR |
TR |
TR |
TR |
TR | Yes | No | |
| (Yes %) (No %) | (yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | ||||
| Action -Policies & procedures | ||||||||
| “Does your facility have a policy that requires prescribes to document in the medical record ordering order entry a dose, duration, and indication for all antibiotic prescriptions?” | 20 (50%) (50%) | 7 (29%) (71%) | 8 (63%) (38%) | 5 (0%) (100%) | 4 (50%) (50%) | 44 | 43% | 57% |
| “Does your facility have facility-specific treatment recommendations, based on national guide lines and local susceptibility, to assist with antibiotic selection for common clinical conditions?” | 20 (55%) (45%) | 7 (14%) (86%) | 8 (25%) (75%) | 5 (40%) (60%) | 4 (0%) (100%) | 44 | 36% | 64% |
| Action -Procedural interventions to improve rationale antibiotic use | ||||||||
| “Is there a formal procedure for all clinicians to review the appropriateness of all antibiotics 48 h after the initial orders (e.g. antibiotic time out)?” | 20 (95%) (5%) | 7 (0%) (100%) | 8 (38%) (63%) | 5 (0%) (100%) | 4 (50%) (50%) | 44 | 55% | 45% |
| “Do specified antibiotic agents need to be approved by a physician or pharmacist prior to dispensing (i.e., pre-authorization) at your facility?” | 20 (90%) (10%) | 7 (86%) (14%) | 8 (63%) (38%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 66% | 34% |
| “Does a physician or pharmacist review courses of therapy for specified antibiotic agents (i.e., prospective audit with feedback) at your facility?” | 20 (80%) (20%) | 7 (29%) (71%) | 8 (75%) (25%) | 5 (0%) (100%) | 4 (75%) (25%) | 44 | 61% | 39% |
Table 3 demonstrates process measures; such as adherence to stewardship policies, specific treatment recommendations and interventions. Outcome measures and antibiotic use was monitored by metrics used in different hospitals. Table 4 shows the reporting and education system in different type of hospitals. Table 4 shows that 48% of hospitals provide specific reports on antibiotic usage to prescribers with 34% prescribers having current antibiograms available. 55% of prescribers received direct, personalized communication about how can improve their antibiotic prescribing and 57% clinicians and other health care professional received stewardship program education.
Table 3.
Tracking.
| Items | Non government teaching |
Government teaching |
Government non-teaching |
Non-government non teaching |
Corporate non-teaching |
Overall |
||
|---|---|---|---|---|---|---|---|---|
| TR |
TR |
TR |
TR |
TR |
TR | Yes | No | |
| (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | ||||
| Tracking -Process measures | ||||||||
| “Does your stewardship program monitor adherence to a documentation policy (dose, duration, and indication)?” | 20 (85%) (15%) | 7 (43%) (57%) | 8 (50%) (50%) | 5 (0%) (100%) | 4 (25%) (75%) | 44 | 57% | 43% |
| “Does your stewardship program monitor adherence to facility-specific treatment recommendations?” | 20 (75%) (25%) | 7 (57%) (43%) | 8 (38%) (63%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 55% | 45% |
| “Does your stewardship program monitor compliance with one of more of the specific interventions in place?” | 20 (80%) (20%) | 7 (29%) (71%) | 8 (25%) (75%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 45% | 55% |
| Tracking -Outcome measures | ||||||||
| “Does your facility track rates of C. diffiile infection?” | 20 (80%) (20%) | 7 (0%) (100%) | 8 (25%) (75%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 41% | 59% |
| “Does your facility produce an antibiotic (cumulative antibiotic susceptibility report?” | 20 (85%) (15%) | 7 (0%) (100%) | 8 (25%) (75%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 36% | 64% |
| Tracking -Monitor antibiotic use by metrics | ||||||||
| “By counts of antibiotic(s) administered to patients per day (Days of Therapy; DOT)?” | 20 (95%) (5%) | 7 (57%) (43%) | 8 (50%) (50%) | 5 (20%) (80%) | 4 (25%) (75%) | 44 | 66% | 34% |
| “By number of grams of antibiotics used (Defied Daily Dose, DDD)?” | 20 (75%) (25%) | 7 (43%) (57%) | 8 (63%) (38%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 52% | 48% |
| “By direct expenditure for antibiotics (purchasing costs)?” | 20 (60%) (40%) | 7 (0%) (100%) | 8 (25%) (75%) | 5 (20%) (80%) | 4 (50%) (50%) | 44 | 39% | 61% |
Table 4.
Reporting and education.
| Items | Non government teaching |
Government teaching |
Government non-teaching |
Non-government non teaching |
Corporate non-teaching |
Overall |
||
|---|---|---|---|---|---|---|---|---|
| TR |
TR |
TR |
TR |
TR |
TR | Yes | No | |
| (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | (Yes %) (No %) | ||||
| Reporting - Inform staff to improve antibiotic use and resistance | ||||||||
| “Does you stewardship program share facility-specific reports on antibiotic use with prescribers?” | 20 (90%) (10%) | 7 (0%) (100%) | 8 (38%) (63%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 48% | 52% |
| “Has a current antibiogram been distributed to prescribers at your facility?” | 20 (65%) (35%) | 7 (0%) (100%) | 8 (25%) (75%) | 5 (0%) (100%) | 4 (0%) (100%) | 44 | 34% | 66% |
| “Do prescribers ever receive direct, personalized communication about how they can improve their antibiotic prescribing?” | 20 (75%) (25%) | 7 (29%) (71%) | 8 (13%) (88%) | 5 (60%) (40%) | 4 (4%) (75%) | 44 | 55% | 45% |
| Education on Antibiotic Stewardship | ||||||||
| “Does your stewardship program provide education to clinicians and other relevant staff on improving antibiotic prescribing?” | 20 (80%) (20%) | 7 (71%) (29%) | 8 25%) (75%) | 5 (40%) (60%) | 4 (0%) (100%) | 44 | 57% | 43% |
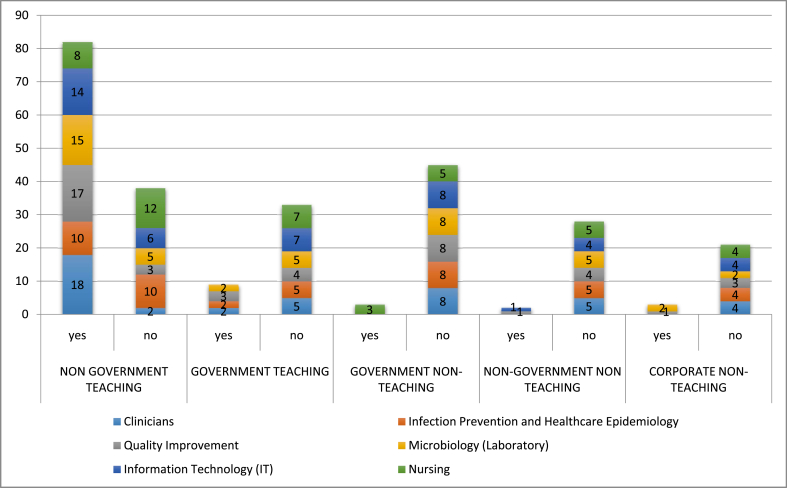
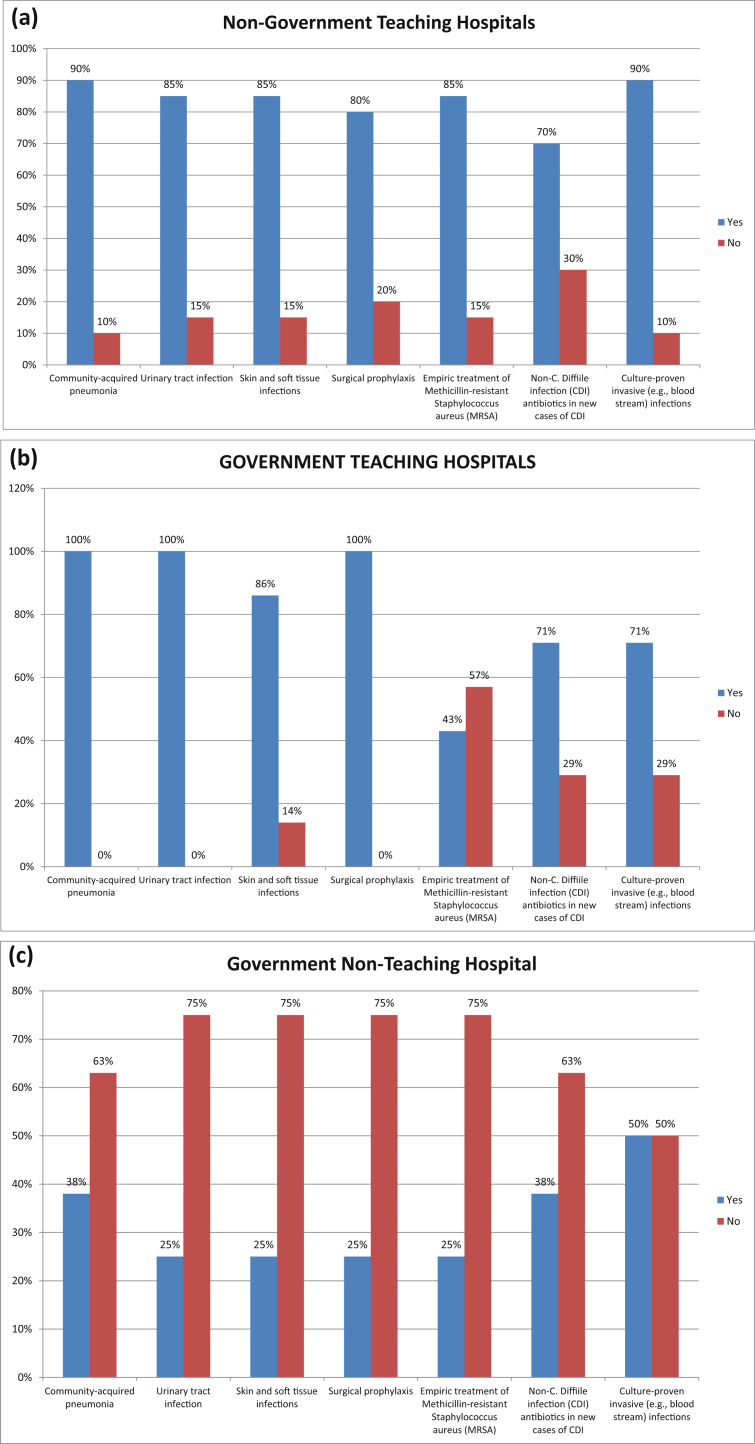
Figure 2 represents the staff from different departments working with stewardship leaders in different types of hospitals in Karachi, to improve rational use of antibiotics. Fig. 3 shows the optimal use of antibiotic in different hospitals of Karachi used to treat common infections. Fig. 3 shows that about of 57% hospitals performed automatic changes from intravenous to oral antibiotic therapy in appropriate situations, 80% hospitals performed dose adjustments in cases of organ dysfunction, and 68% hospitals optimize doses (pharmacokinetics/pharmacodynamics) in the treatment of organisms with reduced susceptibility.
Fig. 2.
Staff working with Stewardship Leaders to improve Anti-biotic Use.
Fig. 3.
Percentage of Optimal Use of Antibiotics for cure common Infections among hospital in Karachi. (a) Non-Government Teaching Hospitals; (b) Government Teaching Hospitals; (c) Government Non-Teaching Hospital; (d) Non-Government Non-Teaching Hospital; (e) Corporate Non-Teaching hospitals.
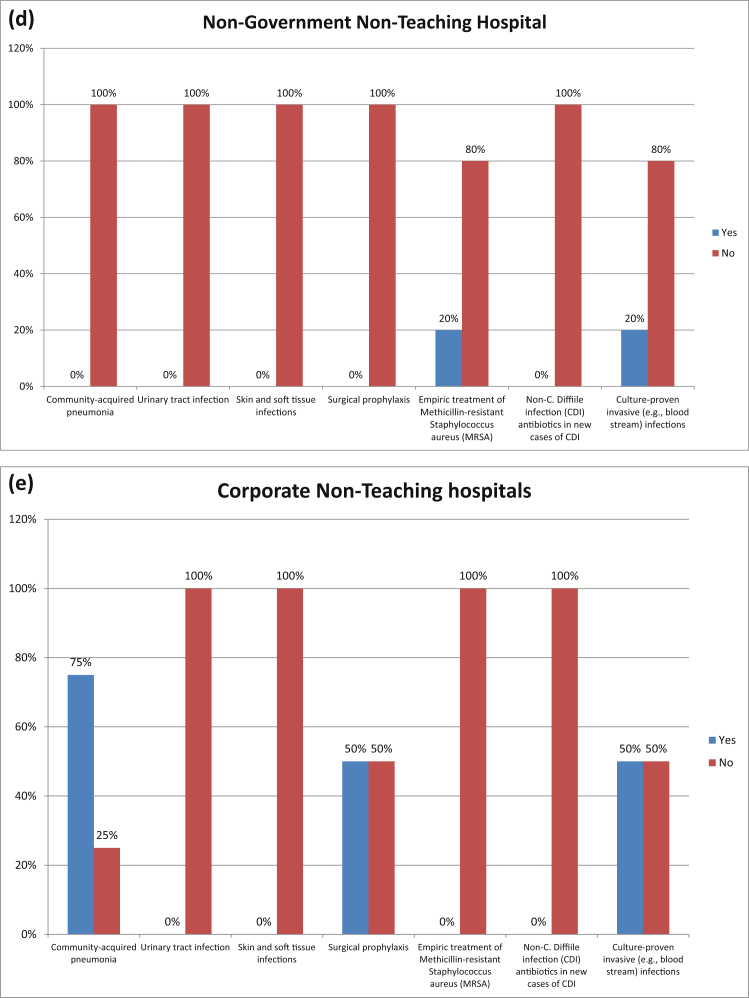
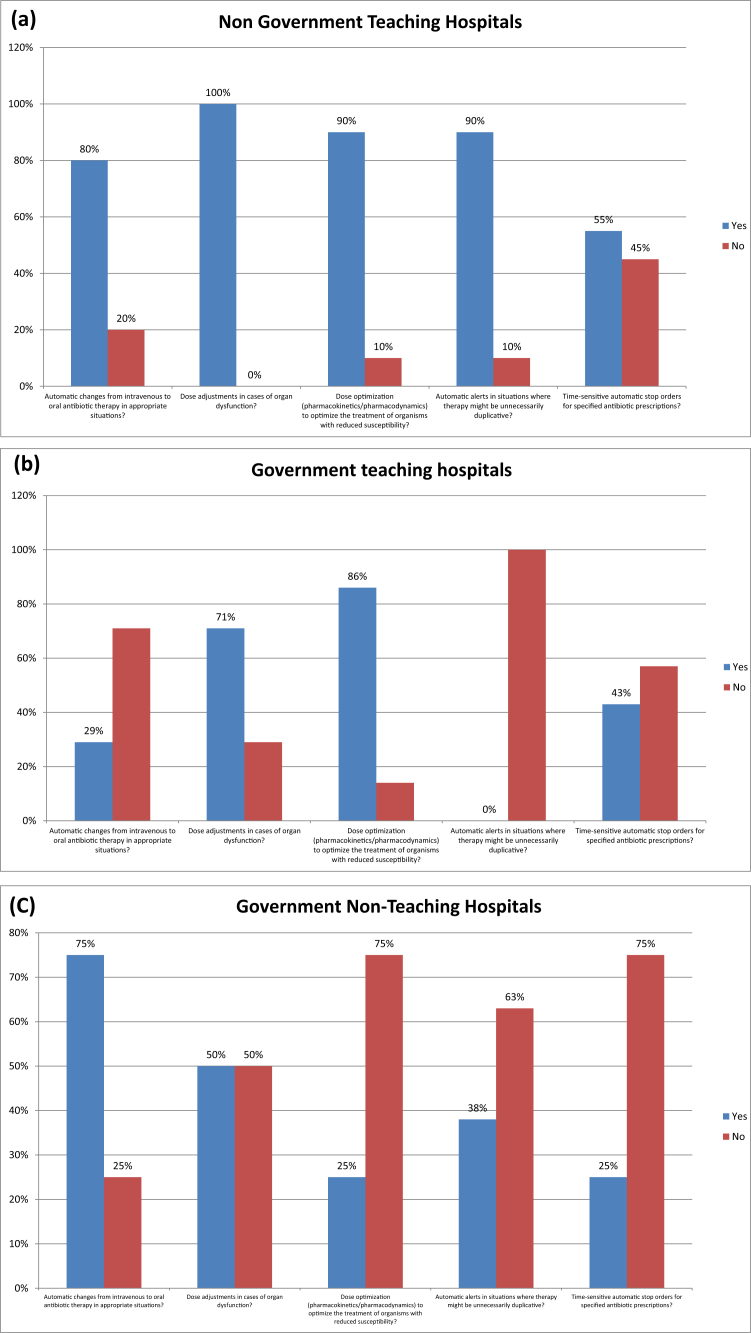
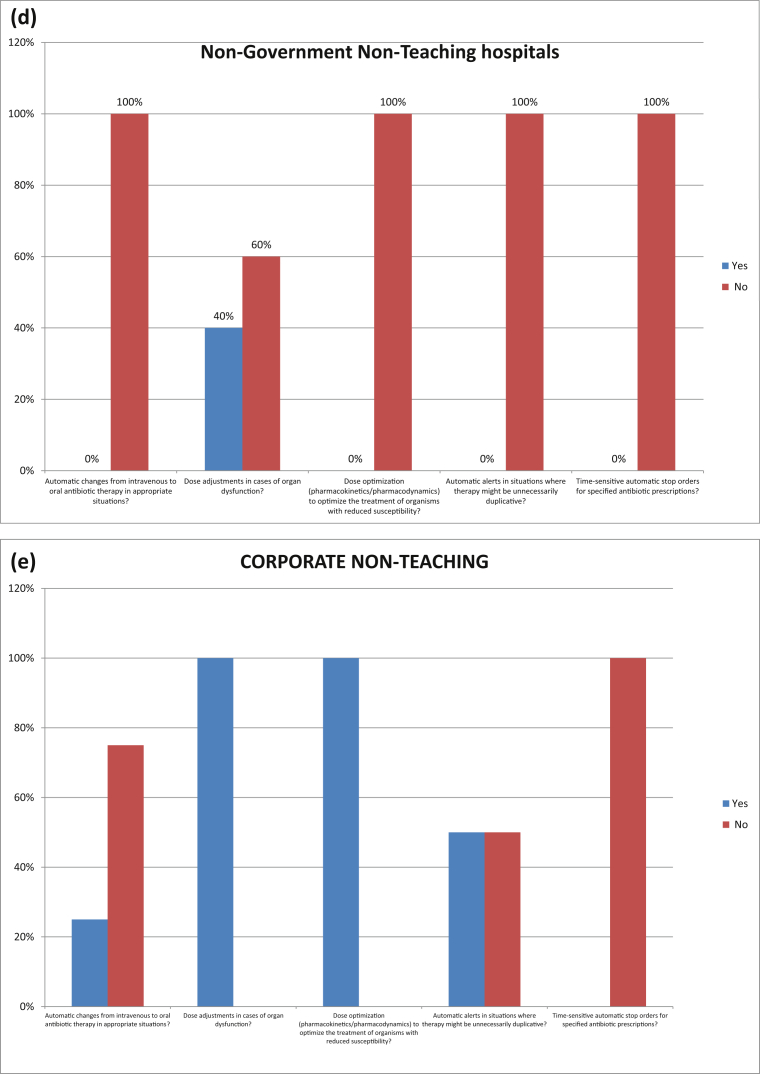
Fig. 4 shows pharmacy driven interventions in different hospitals in Karachi. 52% hospitals ensured automatic alerts in situations where therapy might be unnecessarily duplicative and 36% hospitals performed time-sensitive automatic stop orders for specified antibiotic prescriptions.
Fig. 4.
PHARMACY-DRIVEN INTERVENTIONS: Are the following actions implemented in your facility?. (a) Non-Government Teaching Hospitals; (b) Government Teaching Hospitals; (c) Government Non-Teaching Hospital; (d) Non-Government Non-Teaching Hospital; (e) Corporate Non-Teaching hospitals.
Discussion
This study aimed to determine to which degree hospitals in metropolitan city of Karachi, Pakistan were engaging in stewardship activities. The present study identified that leadership support was not available to implement antimicrobial practices and it was rare to implement integrated ASP. The prime issue is the necessity to preserve the effectiveness of the presently available antibiotics via their usage limiting grounded on basic principles [18]. Official antibiotic stewardship programs are needed to aid society reduce antibiotic resistance by decreasing antibiotic usage which is excessive or inappropriate [19]. Though cost savings from these antibiotic stewardship programs will differ provisionally on the facility size and the degree to which interventions had been implemented, most published studies from higher settings had constantly shown noteworthy annual savings which is around $200,000–$900,000 [20].
Like other developing countries Pakistan has also high antimicrobial use. There are several reasons for this high usage including no restriction on dispensing at pharmacies, physician's preference to use high potency broad-spectrum antibiotics and demand from patients. Tracking of ASP relating to process measures, outcome measures, and monitoring antibiotic usage is shown in Table 3. Some of developed countries have developed organized ASP e.g. United Kingdom [21].
Staff working with stewardship leaders to improve antibiotic usage has been shown in Fig. 2. The constraints demonstrated in our study was regarding support for clinical staff in obtaining expertise in this field, working in a team to improve antibiotic use, a physician leader to oversee outcomes and availability as well as an infectious disease consultation service and infectious disease pharmacist [22].
Fig. 3 showed that the optimal use of antibiotics for common infections. There has been clear increase in prevalence of resistant microorganisms during last two decades. This has resulted in hard to treat infections and delays in the recovery process that increase hospital stay of patients [21]. Findings of this study show that only 9% hospitals in Karachi have implemented ASP whilst the rest are practicing few ASP techniques.
High prevalence of multidrug resistant organisms is a subject of interest globally. As per studies leaded by world health organization in Pakistan, around ninety five percent of participants tested positive for multidrug resistant organisms [23]. This has made the treatment of complex infections such as malaria, tuberculosis and acute respiratory infections in Pakistan more challenging [24].
In this survey, there was recognition within the hospital of antimicrobial stewardship practices and its impact on health outcomes. There are certain limitations highlighted e.g. lack of management commitment, low drug expertise in medical staff, lack of up-to-date policies and procedures, no appropriate tracking of outcome measures and minimal reporting to staff to improve antibiotic use and resistance. One nationwide survey conducted on antimicrobial stewardship practices in USA noted that healthcare providers understand importance of the stewardship activities and give formal recognition to stewardship programs in this critical phase, where antimicrobial resistance is increasing tremendously.
There is recognition of ASP barriers that remain to implementation that are cumbersome to overcome. This study highlights that staff limitations and budget allocation as major barriers in implementation of ASP. Institutional commitment to stewardship was also low despite that fact that antimicrobial resistance is global problem [22]. A study conducted in 67 countries from 6 continents of world including Africa, Asia, Europe, North America, Oceania, and South America showed that ASP existed in 52% countries while in 4% they were planned [25].
The study had certain limitations. Firstly, respondents had been self-chosen and the data entry method had not been validated. Additionally, enrolment in the study occurred either through personal contacts of the authors or via professional associations. Secondly, the questionnaire interpretation and definitions used may not been consistent or clear between respondents.
Conclusion
This study showed that all the hospitals in one setting failed to comply with all the guidelines. It has been concluded that efforts should be made to design ASP at each hospital and implemented through suitable policies and procedures.
Antibiotics save the lives of millions of peoples but its inappropriate use can lead to antibiotic resistance and hence, limit resources. The goal of ASP is that patients receive the correct antibiotics, with correct dose and duration and at correct time. Core elements of ASP provide the basis for all health care providers to improve the antibiotic prescribing and improve patient outcomes.
References
- 1.Ansari F., Erntell M., Goossens H., Davey P. Group EIHCS the European surveillance of antimicrobial consumption (ESAC) point-prevalence survey of antibacterial use in 20 European hospitals in 2006. Clin Infect Dis. 2009;49:1496–1504. doi: 10.1086/644617. [DOI] [PubMed] [Google Scholar]
- 2.Hecker M.T., Aron D.C., Patel N.P., Lehmann M.K., Donskey C.J. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 3.Bedini A., De Maria N., Del Buono M., Bianchini M., Mancini M., Binda C., Brasacchio A. Antimicrobial stewardship in a gastroenterology department: impact on antimicrobial consumption, antimicrobial resistance and clinical outcome. Dig Liver Dis. 2016;48:1142–1147. doi: 10.1016/j.dld.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Dellit T.H., Owens R.C., McGowan J.E., Gerding D.N., Weinstein R.A., Burke J.P. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 5.Broom J.K., Broom A.F., Kirby E.R., Gibson A.F. Post JJ Clinical and social barriers to antimicrobial stewardship in pulmonary medicine: a qualitative study. Am J Infect Contr. 2017;45:911–916. doi: 10.1016/j.ajic.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel R.P. The antibiotic pipeline—challenges, costs, and values. N Engl J Med. 2004;351:523–526. doi: 10.1056/NEJMp048093. [DOI] [PubMed] [Google Scholar]
- 7.Barber K.E., Bell A.M., Travis King S., Parham J.J., Stover K.R. Impact of piperacillin-tazobactam shortage on meropenem use: implications for antimicrobial stewardship programs. Braz J Infect Dis. 2016;20:631–634. doi: 10.1016/j.bjid.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDougall C., Polk R.E. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke J.P. Infection control–a problem for patient safety. N Engl J Med. 2003;348:651. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence K.L., Kollef M.H. Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med. 2009;179:434–438. doi: 10.1164/rccm.200809-1394CP. [DOI] [PubMed] [Google Scholar]
- 11.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 12.Brown E.M., Nathwani D. Antibiotic cycling or rotation: a systematic review of the evidence of efficacy. J Antimicrob Chemother. 2005;55:6–9. doi: 10.1093/jac/dkh482. [DOI] [PubMed] [Google Scholar]
- 13.Lutters M., Harbarth S., Janssens J.P., Freudiger H., Herrmann F., Michel J.P. Effect of a comprehensive, multidisciplinary, educational program on the use of antibiotics in a geriatric university hospital. J Am Geriatr Soc. 2004;52:112–116. doi: 10.1111/j.1532-5415.2004.52019.x. [DOI] [PubMed] [Google Scholar]
- 14.Brink A.J., Messina A.P., Feldman C., Richards G.A., Becker P.J., Goff D.A. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16:1017–1025. doi: 10.1016/S1473-3099(16)30012-3. [DOI] [PubMed] [Google Scholar]
- 15.Poteete C. Scaletta JM Antimicrobial stewardship in Kansas: results from a statewide survey. Am J Infect Contr. 2017;45:42–45. doi: 10.1016/j.ajic.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 16.CDC . US Department of Health and Human Services, CDC; Atlanta, GA: 2014. Core Elements of Hospital Antibiotic Stewardship Programs.http://www.cdc.gov/getsmart/healthcare/implementation/core-elements.html Available at. [Google Scholar]
- 17.Pakistan Economic Survey . Ministry of Finance; Islamabad: 2016. Economic Advisor's Wing.http://www.finance.gov.pk/survey/chapters_17/Pakistan_ES_2016_17_pdf.pdf 2016 -2017. [Google Scholar]
- 18.Burgess L.H., Miller K., Cooper M., Moody J., Englebright J., Septimus E. Phased implementation of an antimicrobial stewardship program for a large community hospital system. Am J Infect Contr. 2019;47:69–73. doi: 10.1016/j.ajic.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Spellberg B., Srinivasan A., Chambers H.F. New societal approaches to empowering antibiotic stewardshipnew societal approaches for empowering antibiotic stewardshipnew societal approaches for empowering antibiotic stewardship. JAMA. 2016;315:1229–1230. doi: 10.1001/jama.2016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Contr. 2019;8:35. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner S.J., Pryer J.A., Duffy E.J. Survey of antimicrobial stewardship practices in public hospitals in New Zealand district health boards. Infection. 2017;20:100. [PubMed] [Google Scholar]
- 22.Doron S., Nadkarni L., Price L.L., Lawrence P.K., Davidson L.E., Evans J. A nationwide survey of antimicrobial stewardship practices. Clin Ther. 2013;35:758–765. doi: 10.1016/j.clinthera.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Dawn News Paper . Dawn The Jung Group; Pakistan: 2016. Antibiotic resistance. [Google Scholar]
- 24.Sarwar M.R., Saqib A., Iftikhar S., Sadiq T. Knowledge of community pharmacists about antibiotics, and their perceptions and practices regarding antimicrobial stewardship: a cross-sectional study in Punjab, Pakistan. Infect Drug Resist. 2018;11:133. doi: 10.2147/IDR.S148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard P., Pulcini C., Levy Hara G., West R.M., Gould I.M., Harbarth S. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2014;70:1245–1255. doi: 10.1093/jac/dku497. [DOI] [PubMed] [Google Scholar]