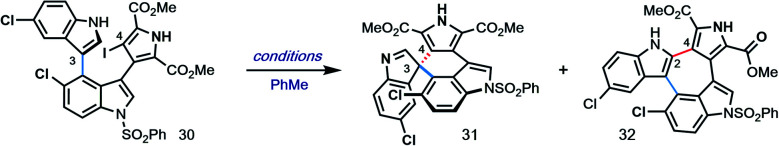
Optimization of Pd-catalyzed spirocyclizationa.
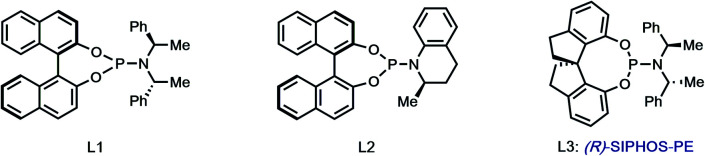
| ||||||
|---|---|---|---|---|---|---|
| Entry | Pd/ligand | Base | T (°C) | 31 : 32b | Yield (%)c | eed |
| 1 | Pd-PEPPSI-IPr | Cs2CO3 | 115 | 1.8 : 1 | 55 | n/a |
| 2 | [Pd]/L1 | Cs2CO3 | 90 | 1 : 1.1 | 11 | −14 |
| 3 | [Pd]/L2 | Cs2CO3 | 90 | 1.7 : 1 | 14 | 4 |
| 4 | [Pd]/L3 | Cs2CO3 | 90 | 1 : 1.5 | 9 | 75 |
| 5 | [Pd]/L3e | Cs2CO3/Ag2CO3f | 105 | 1 : 2 | 8 | 83 |
| 6 | [Pd]/L3e | Cs2CO3/Ag2CO3f | 80 | 1 : 2.1 | 21 | 86 |
| 7 | [Pd]/L3g | Cs2CO3/Ag2CO3f | 70 | 1 : 2.2 | 14 | 98 |
| 8 | [Pd]/L1e | Cs2CO3/Ag2CO3f | 70 | 1 : 1.8 | 6 | −53 |
| ||||||
[Pd] = [Pd(allyl)Cl]2; standard conditions: Pd source (10 mol%), ligand (15 mol%), base (1.5 equiv.).
Determined by 1H NMR analysis of the crude reaction mixture.
Yield of isolated 31.
Determined by HPLC analysis.
[Pd]/ligand (30/45 mol%) prestirred in PhMe for 1 h.
2.5 equiv. each.
[Pd]/ligand (40/60 mol%).
