Table 3:
Structures of 12-epi-hapalindole U and 12-epi-hapalindole C derivatives produced by FamC1 and HpiC1 from unnatural cis-indole isonitrile or cis-indole nitrile substrates.c
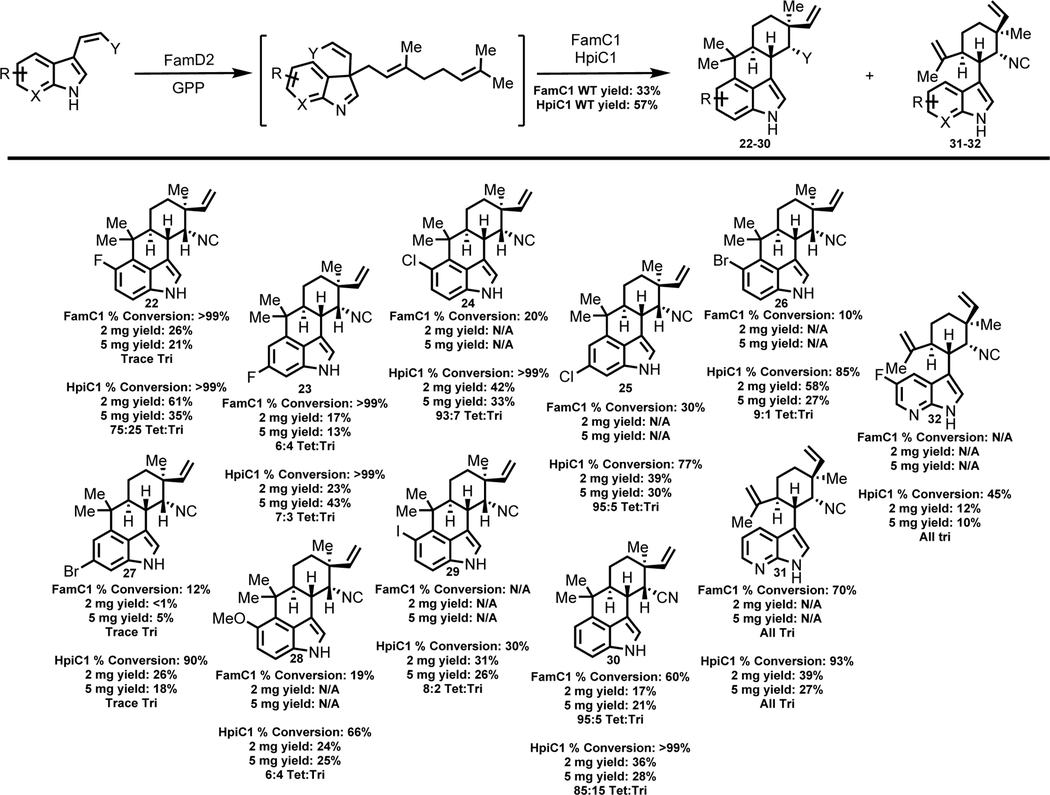
|
Percent conversions, isolated yield values and tetracyclic:tricyclic ratio (ratio estimated by NMR and/or HPLC) are shown below each derivative. N/A=Derivative was either not produced by FamC1 or was not screened further in this study due to enhanced versatility of HpiC1. HPLC conversion values determined after 4 hrs in 100 μL reactions. Isolated yield values from overnight reactions.
