Figure 5.
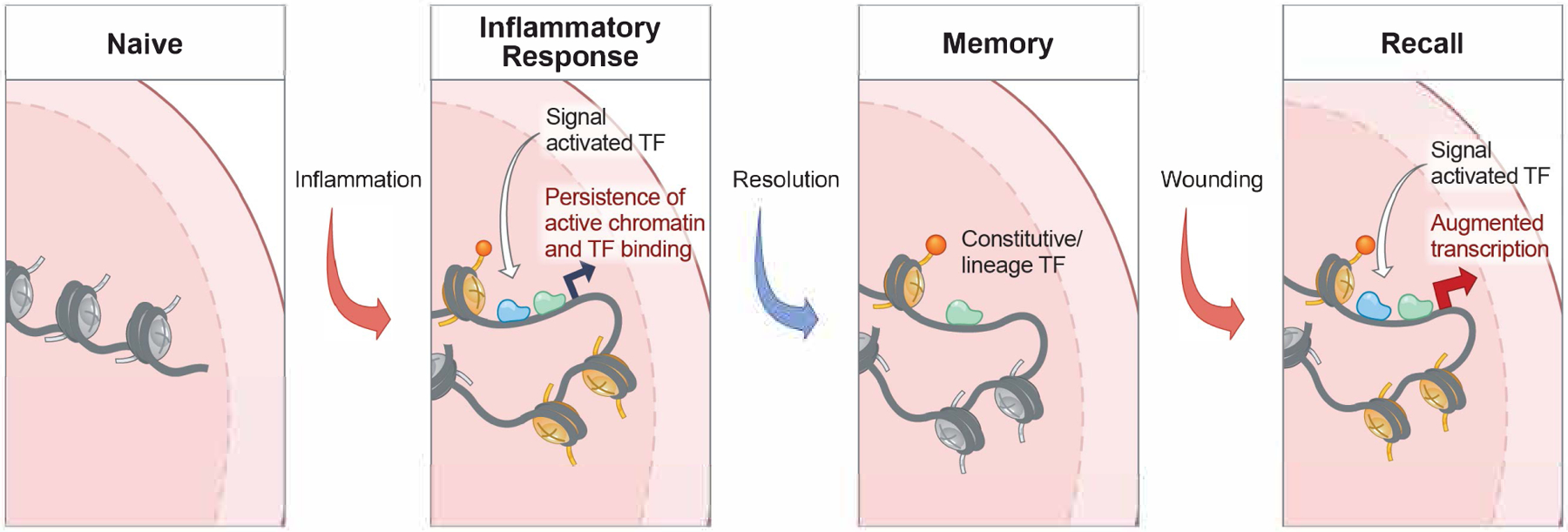
Model of the acquisition of cell intrinsic memory and augmented function in barrier tissue cells.
During exposure to inflammation, a barrier tissue cell experiences the transient activity of signal activated transcription factors such as pSTAT3 and NFkB. In a model still untested, these transcription factors open inflammation response gene chromatin, possibly providing accessibility for the binding of native transcription factors and/or histone marks. Following resolution of the inflammatory state, these homeostatic chromatin marks could then leave behind a durable epigenetic memory of inflammation. In barrier epithelial stem cells such as EpdSCs, most of these genes exist in a poised (accessible) but transcriptionally dormant state (Naik et al., 2017). Upon rechallenging, this poise state then becomes rapidly re-activated, presumably though easier accessibility of inflammation-activated transcription factors, rapidly fueling transcription and augmented growth and tissue repair. How memory endows cells with the ability to respond more rapidly to different inflammatory experiences remains a mystery, even in current models such as this one.
