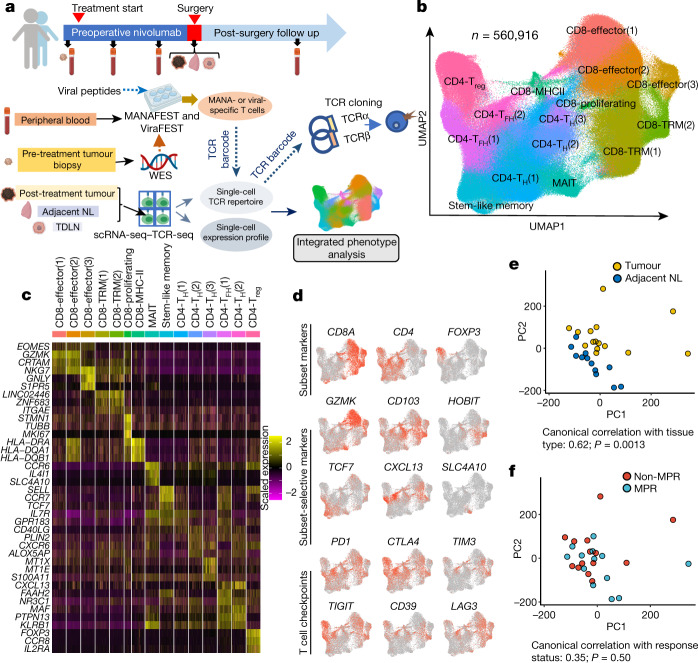
Fig. 1. Profiling single T cells in NSCLC treated with neoadjuvant PD-1 blockade.
Twenty patients with resectable NSCLC were treated with two doses of PD-1 blockade before surgical resection. a, An overall schematic of the clinical trial, biospecimen collection (top) and study design (bottom). scRNA-seq–TCR-seq was performed on T cells isolated from resected tumour (n = 15), adjacent normal lung (NL; n = 12), TDLN (n = 3), and a resected brain metastasis (n = 1) from patients with NSCLC treated with two doses of neoadjuvant anti-PD-1 (bottom). The MANAFEST and ViraFEST assays were used to identify MANA- and viral (EBV and influenza)-specific TCRs, respectively. WES, whole-exome sequencing. b, UMAP projection of the expression profiles of the 560,916 T cells that passed quality control. Immune cell subsets, defined by 15 unique clusters, are annotated and marked by colour code. c, Relative expression of the top-3 most differentially expressed genes. Five-thousand cells (or all cells in the cluster if the cluster size was fewer than 5,000 cells) were randomly sampled from each cluster for visualization. MAIT, mucosal-associated invariant T cells; TFH, T follicular helper cells; Treg, regulatory T cells. d, Expression of T cell subset-defining genes, T cell subset-selective genes and major T cell checkpoint genes. CD39 is also known as ENTPD1. e, PCA of cell-cluster-level pseudobulk gene expression for individual samples for tumour (yellow, n = 15) and adjacent normal lung (dark blue, n = 12). One-sided permutation test. f, PCA of cell-cluster-level pseudobulk gene expression for non-MPR (red, n = 9) and MPR (light blue, n = 6) tumours. One-sided permutation test.