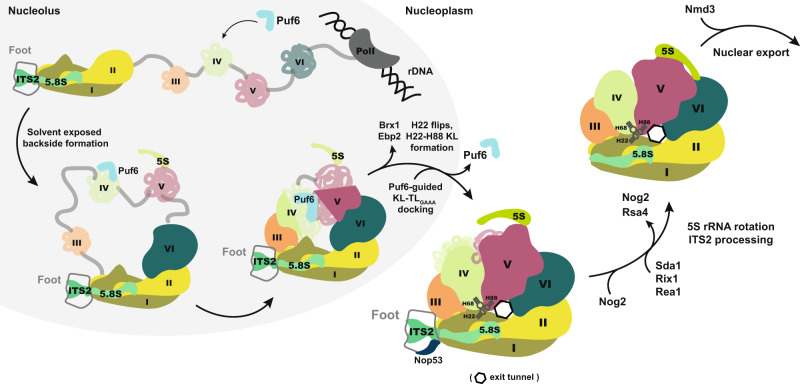
Fig. 8. Schematic for Puf6 function during 60S assembly.
The 27S rRNA precursor co-transcriptionally associates with assembly factors/r-proteins and folds into rRNA-independent domains. The solvent-exposed side (domains I, II, and VI) establishes initial tertiary contacts which are followed by gradual compaction of domains III–V that form the catalytic subunit interface within the 60S subunit. Puf6 (light blue) is co-transcriptionally recruited to H68 within domain IV where it awaits the formation of KL between H22-H88. Release of assembly factors Brx1-Ebp2 enables a conformational change of H22 and its productive engagement with H88, forming a KL—the receptor for H68-TLGAAA. Puf6 ushers H68 docking onto the pre-formed KL that stabilizes the subunit interface serving a binding platform for 5S RNP maturation factors (Sda1, Rix1, Rea1) and export driving machinery (Nog2, Nmd3).