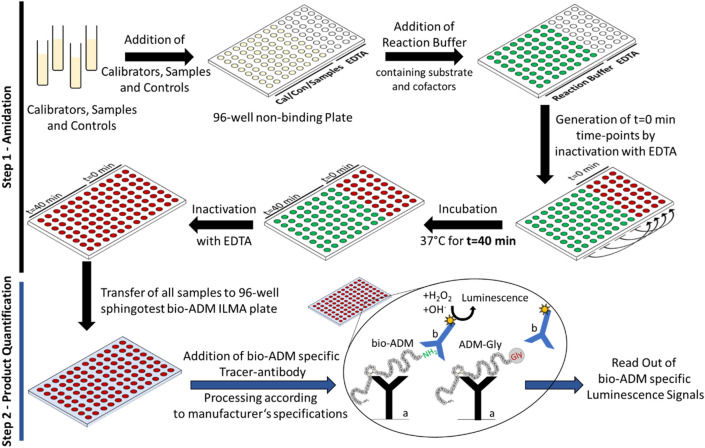
Figure 1.
Schematic representation of the AMA Assay. Step 1—amidation: calibrators, controls and samples are dispensed in double determination as indicated (yellow) into a non-binding 96-well plate. The reaction is started by addition of reaction buffer to calibrators, controls and samples (green) and a t = 0 min time-point is generated immediately by transferring equal volumes of each double determination into EDTA prefilled wells (red). The generated t = 0 min time-points are single determinations. After incubation at 37 °C for 40 min an inactivation of remaining samples with EDTA is performed. Step 2—product quantification: following 37 °C incubation and inactivation, all samples are transferred into the sphingotest bio-ADM Assay 96-well immunoluminometric assay (ILMA) plate. The bio-ADM assay is processed according to manufacturer’s specifications. Both, ADM-Gly and bio-ADM are bound by the solid-phase antibody (a). The MACN labelled tracer-antibody (b) specifically recognizes the c-terminally amidated bio-ADM and does not react with ADM-Gly. Following incubation, unbound tracer-antibody is washed away and bio-ADM specific luminescence signals are detected using a standard plate luminometer. The figure was created with Microsoft PowerPoint 2016, version 2105.