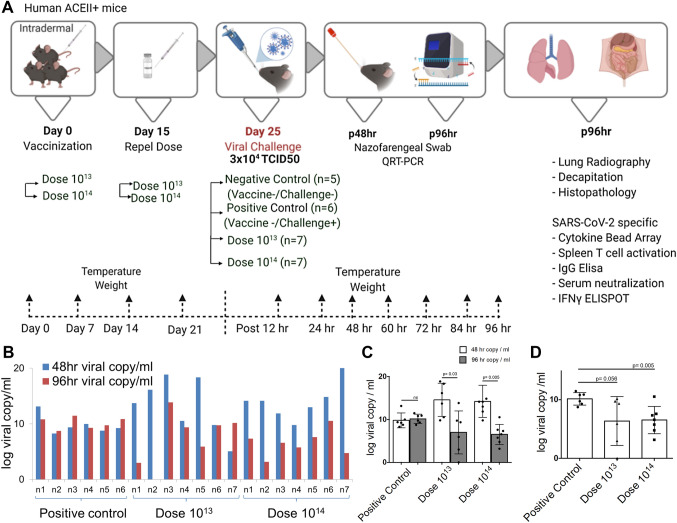
Figure 3.
Challenge test with OZG-38.61.3 vaccinated humanized ACEII+ mice. (A) Representation of in vivo experimental setup of the challenge test. (A) Mice were allocated into 4 groups, a negative control group (n = 5), a positive control group (n = 6), and 2 different intradermally vaccinated groups (dose 1013 and dose 1014, n = 7 per group). A booster dose of dose 1013 and dose 1014 vaccine was administered on day 15. After 25 days of vaccination, the mice were intranasally infected with a 3 × 104 TCID50 dose of SARS-CoV-2. Biopsy samples, spleen T cells, and serum were collected after euthanization at 96 h. (B) The bar graph showing SARS-CoV-2 viral RNA copy number in log scale per ml of the nasopharyngeal samples collected from each mouse at 48 h and 96 h post-challenge that were either vaccinated with dose 1013 (n = 7), dose 1014 (n = 7), or without vaccination group (positive control; n = 6). (C) The bar graph shows the mean value of viral RNA copy in log scale per ml at 48 h and 96 h post-challenge. (D) The bar graph showing a comparison of viral RNA copy number at 96 h between vaccinated and positive control groups.