Figure 1.
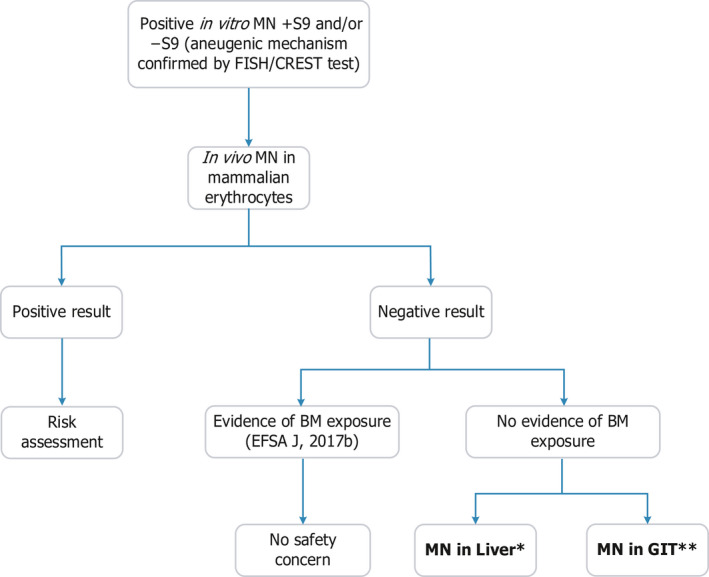
- BM: bone marrow; GIT: gastrointestinal tract; MN: micronucleus test. *: In the process of being considered for the development of an OECD TG (Kirkland et al., 2019). **: For a positive in vitro MN test in the absence of S9, and after negative results in an in vivo MN with no evidence of BM exposure, a GIT MN assay would be appropriate, but more work is required for an OECD guideline (see text).
