Abstract
Objective: We aimed to compare the efficacy of microsurgical clipping of intracranial aneurysms with that of arterial embolization in the treatment of ruptured anterior circulation aneurysms. Methods: The clinical data of 68 patients treated in our hospital for ruptured anterior circulation aneurysms between January 2017 and March 2020 were analyzed retrospectively. According to the surgical methods, the patients were divided into two groups: the microsurgical clipping group (30 cases) and the arterial embolization group (38 cases). The following markers were compared between the two groups: Hunt-Hess classification (HHC) grading, aneurysm occlusion rate, and incidence of postoperative complications, length of hospital stay, hospitalization cost, and the scores of the Glasgow Outcome Scale, Modified Rankin Scale, and Barthel Index during the 6-months follow-up after hospital discharge. Results: The cases of HHC grade I and II increased in both groups at hospital discharge (both P<0.05), and there was no intergroup difference in this marker (P>0.05). The complete occlusion rate in the microsurgical clipping group was higher than that in the arterial embolization group (P<0.05). Compared with the microsurgical clipping group, the arterial embolization group had shorter length of hospital stay and higher hospitalization cost (both P<0.05). There was no difference in the total incidence of postoperative complications between the two groups (P>0.05). However, the arterial embolization group had lower incidence of intracranial infection and higher incidence of vasospasm than the microsurgical clipping group (both P<0.05). During the follow-up, the arterial embolization group had better results in terms of the Modified Rankin Scale and Barthel Index results and had more patients with GOS score of 5 points than the microsurgical clipping group (all P<0.05). Conclusion: Both microsurgical clipping of intracranial aneurysms and arterial embolization can effectively treat ruptured anterior circulation aneurysms, and the short-term efficacy achieved by these two methods is similar. Compared with microsurgical clipping of intracranial aneurysms, arterial embolization can lead to shorter hospitalization, lower incidence of intracranial infection, and better patients’ prognosis and quality of life after the operation. However, the microsurgical clipping of intracranial aneurysms can achieve higher complete occlusion rate, lower incidence of vasospasm, and lower hospitalization cost than arterial embolization.
Keywords: Intracranial aneurysm, microsurgical clipping, arterial embolization
Introduction
Intracranial aneurysm is a common disease and considered to be the major cause of subarachnoid hemorrhage. It is estimated that 85% of intracranial aneurysms occur in the anterior circulation. Once the intracranial aneurysm is ruptured and bleeding occurs, the patients can have severe headache, go into a coma, or even die. Even if the patients survive, they can still have various disabilities and physiological dysfunctions. Clinically, the total mortality of intracranial aneurysms is over 25% [1]. Therefore, how to improve the prognosis of patients with ruptured anterior circulation aneurysms and reduce mortality has become an essential medical research topic.
Microsurgical clipping of intracranial aneurysms and arterial embolization have been proven to be effective methods for treating ruptured anterior circulation aneurysms and have been widely applied in clinical practice [2-5]. The surgical intracranial clipping technique started in the 1970s. With the development of microsurgery, the disability and mortality rates after microsurgical clipping have been declining each year [6]. However, compared with arterial embolization, microsurgical clipping can cause greater surgical trauma and higher incidence of postoperative complications such as delayed cerebral ischemia [7]. In recent years, arterial embolization has developed rapidly and is considered to have a therapeutic effect similar to that of microsurgical clipping [8]. Moreover, arterial embolization can lead to less surgical trauma and shorten postoperative recovery. However, intracranial embolus detachment, vasospasm, and secondary hemorrhage may occur during arterial embolization, and the recurrence rate of the aneurysm after this procedure is high [9]. So far, there is still no conclusion regarding which surgical method can achieve a better outcome and bring lower risks [10].
In the present study, we aimed to further compare the efficacy of microsurgical clipping of intracranial aneurysms with that of arterial embolization in the treatment of ruptured anterior circulation aneurysms, in an effort to provide more theoretical basis for the clinical treatment of this disease.
Materials and methods
Participants
The clinical data of 68 patients treated in our hospital for ruptured anterior circulation aneurysms between January 2017 and March 2020 were analyzed retrospectively. The study was approved by the Ethics Committee of our hospital.
Inclusion criteria
(1) patients who had complete clinical data; (2) patients who were diagnosed with ruptured anterior circulation aneurysm by CT or digital subtraction angiography (DSA); (3) patients whose onset started less than 72 hours prior to treatment; (4) patients whose aneurysm diameter was no more than 25 mm; (5) patients whose Hunt-Hess classification (HHC) grade was II-IV before the operation; (6) patients who met the indications for microsurgical clipping or arterial embolization at hospital admission and underwent microsurgical clipping or arterial embolization.
Exclusion criteria
(1) patients with incomplete clinical data; (2) patients with coagulation disorders or active bleeding; (3) patients who had dysfunctions of important organs; (4) patients with severe infection; (5) patients with intracranial vasculitis or congenital vascular malformation; (6) patients with mental disease or cognitive dysfunction; (7) patients with malignant tumors.
Upon hospital admission, all patients and their family members were informed of the effects and potential risks of microsurgical clipping and arterial embolization and were asked to choose between the two procedures. A total of 30 patients underwent microsurgical clipping of intracranial aneurysms (the microsurgical clipping group) and 38 underwent arterial embolization (the arterial embolization group).
Methods
Treatment methods
The microsurgical clipping of intracranial aneurysms was performed through pterional approach after anesthesia. The lateral fissure was separated under the microscope to expose the parent artery and aneurysm neck. The aneurysm neck was separated bluntly, and the aneurysm was clipped with an aneurysm clip. A suture was performed after the bleeding stopped.
During the arterial embolization, the femoral artery was punctured using the Seldinger technique after anesthesia. The F6 arterial sheath was inserted and whole body heparinization was performed. The catheter was passed over the guidewire into the tumor feeding arteries and then observed using DSA. The arteries were embolized with micro coils followed by DSA reexamination. If there was no residual contrast agent in the aneurysm, the guidewire and catheter were removed. The sheath was removed six hours after the operation. Pressure bandage was applied on the wound to gain hemostasis.
Efficacy evaluation
HHC system was used to evaluate the neurological function of patients [11]. The classification had the following five grades: grade I = asymptomatic or mild headache and neck stiffness; grade II = moderate to severe headache, neck stiffness, no neurologic deficit except cranial nerve palsy; grade III = drowsy, disturbance of consciousness or mild focal neurological deficit; grade IV = stuporous, moderate to severe hemiparesis, and possibly early decerebrate rigidity and vegetative disturbances; grade V = deep coma, decerebrate rigidity, moribund.
Glasgow Outcome Scale (GOS) was used to assess the prognosis of the patients [12]. The scale was a 5-level score, with higher scores indicating better prognosis: 5 = good recovery (the patient resumed normal activities buy may have minor residual problems); 4 = moderately disabled (the patient was independent but disabled); 3 = severely disabled (the patient required others for daily support due to disability); 2 = vegetative state; 1 = dead.
Modified Rankin Scale (mRS) was used to measure the level of independence in activities of daily living (ADL) [13]. The scale was a 5-level score, with lower scores indicating better independence in activities of daily living.
Barthel Index (BI) was used to evaluate the patients’ performance in ADL [14]. The scale included 10 activities: feeding, personal toileting, bathing, dressing and undressing, getting on and off a toilet, controlling bladder, controlling bowel, moving from wheelchair to bed and returning, walking on a level surface (or propelling a wheelchair if unable to walk) and ascending and descending stairs. The total score was 100 points. The higher the score, the stronger performance in ADL.
Outcome measures
Main outcome measures
The HHC grading was compared between the two groups after the treatment. The GOS, mRS and BI scores were compared between the two groups during the 6-month follow-up.
Secondary outcome measures
The aneurysm occlusion rate after the operation, hospital length of stay, and postoperative complications were compared between the two groups [15]. In the microsurgical clipping group, the patients were re-examined with computed tomography angiography (CTA) 7 days after the operation to determine if the clipping was complete or partial. In the arterial embolization group, the patients were re-examined with magnetic resonance angiography (MRA). The assessment criteria by MRA were as follows: 1) if the aneurysms were not visible, the embolization rate was 100%; 2) if a small residual aneurysm neck was present, the embolization rate was 95%; 3) if the residual aneurysm neck was present, the embolization rate was 90%; 4) if the residual aneurysm neck and a small tumor body were present, the embolization rate was 80%; 5) if the residual tumor body was present, the embolization rate was <80%. In this study, 95%-100% embolization rate was considered as complete occlusion.
Statistical analysis
SPSS 26.0 was applied for statistical analysis. Measurement data are expressed as mean ± sd. Comparison between the two groups was performed by independent t-test, and comparison between pre- and post-treatment within a group was performed by paired samples t-test. Count data are presented as number or percentage and were examined by χ2 test. If the expected count was over 20%, the count data were examined by Fisher’s exact test. P<0.05 indicated a statistically significant difference.
Results
Baseline data
There were no differences in the baseline data between the two groups as shown in Table 1 (all P>0.05).
Table 1.
Baseline data in the two groups
| Baseline data | Microsurgical clipping group | Arterial embolization group | χ2/t | P |
|---|---|---|---|---|
| Case (n) | 30 | 38 | ||
| Age (year) | 50.1±7.8 | 52.5±7.5 | 0.853 | 0.396 |
| Gender (male/female) | 12/18 | 14/24 | 0.071 | 0.790 |
| BMI (kg/m²) | 25.74±2.77 | 25.26±2.81 | 1.029 | 0.469 |
| Smoking | 15 | 12 | 2.376 | 0.123 |
| Drinking | 13 | 17 | 0.295 | 0.587 |
| Presence of high blood pressure | 15 | 17 | 0.186 | 0.666 |
| Presence of diabetes | 18 | 24 | 0.071 | 0.790 |
| Presence of hypercholesterolemia | 16 | 19 | 0.075 | 0.785 |
| Presence of cardiovascular disease | 11 | 15 | 0.056 | 0.813 |
| Hunt-Hess classification | 0.118 | 0.990 | ||
| Grade I | 10 | 14 | ||
| Grade II | 10 | 12 | ||
| Grade III | 7 | 8 | ||
| Grade IV | 3 | 4 | ||
| Aneurysm diameter (mm) | 0.076 | 0.963 | ||
| Diameter <5 mm | 14 | 19 | ||
| 5 mm ≤ diameter <15 mm | 10 | 12 | ||
| 15≤ diameter <25 mm | 6 | 7 | ||
| Number of aneurysms | 0.369 | 0.543 | ||
| Single tumor | 12 | 18 | ||
| Multiple tumors | 18 | 20 |
Note: BMI: body mass index.
HHC grading in the two groups
The number of patients with HHC grade I-II in each group at hospital discharge was higher than that at hospital admission (both P<0.05). There was no intergroup difference in HHC grading at discharge (P>0.05). See Table 2.
Table 2.
HHC grade in the two groups before and after treatment (n, %)
| Group | HHC grade I | HHC grade II | HHC grade III | HHC grade IV | HHC graade I-II |
|---|---|---|---|---|---|
| Microsurgical clipping group (n=30) | |||||
| At admission | 7 (23.33) | 15 (50.00) | 6 (20.00) | 2 (6.67) | 22 (73.33) |
| At discharge | 10 (33.33) | 16 (53.33) | 3 (10.00) | 1 (3.33) | 28 (93.33)* |
| Arterial embolization group (n=38) | |||||
| At admission | 7 (18.42) | 19 (50.00) | 10 (26.32) | 2 (5.26) | 26 (68.42) |
| At discharge | 9 (23.68) | 22 (57.89) | 6 (15.79) | 1 (2.63) | 31 (81.58)* |
Note: Compared with the same group at hospital admission;
P<0.05.
HHC: Hunt-Hess classification.
Postoperative complications in the two groups
There was no difference in the total incidence of postoperative complications between the two groups (P>0.05). Compared with the arterial embolization group, the microsurgical clipping group had a higher incidence of intracranial infection and a lower incidence of vasospasm (both P<0.05, Table 3).
Table 3.
Incidence of postoperative complications in the two groups (n, %)
| Group | Intracranial infection | Vasospasm | Hydrocephalus | Rehemorrhage | Total incidence of complications |
|---|---|---|---|---|---|
| Microsurgical clipping group (n=30) | 6 (20.00) | 1 (3.33) | 2 (6.67) | 1 (3.33) | 10 (33.33) |
| Arterial embolization group (n=38) | 1 (2.63)* | 8 (21.05)* | 1 (2.63) | 3 (7.89) | 13 (34.21) |
Note: Compared with the microsurgical clipping group;
P<0.05.
Aneurysm occlusion rate in the two groups after the operation
Based on the CTA results in the microsurgical clipping group and the MRA results in the arterial embolization group, the complete clipping rate in the microsurgical clipping group was higher than the complete embolization rate in the arterial embolization group (P<0.05). See Table 4.
Table 4.
Aneurysm occlusion in the two groups after the operation (n, %)
| Group | Complete clipping | Partial clipping | Complete embolization | Presence of residual aneurysm neck/tumor body | Complete clipping/complete embolization rate (%) |
|---|---|---|---|---|---|
| Microsurgical clipping group (n=30) | 29 (96.67) | 1 (3.33) | 96.67 | ||
| Arterial embolization group (n=38) | 32 (84.21) | 5 (15.79) | 84.21* |
Note: Compared with the microsurgical clipping group;
P<0.05.
Length of hospital stay and cost of hospitalization in the two groups
Compared with the arterial embolization group, the microsurgical clipping group had a longer length of hospital stay and lower cost of hospitalization (both P<0.001). See Table 5.
Table 5.
Length of hospital stay and cost of hospitalization in the two groups (x̅ ± sd)
| Group | Length of hospital stay (day) | Cost of hospitalization (10,000 RMB) |
|---|---|---|
| Microsurgical clipping group (n=30) | 18.98±2.78 | 1.94±0.28 |
| Arterial embolization group (n=38) | 13.24±2.52*** | 4.92±0.71*** |
Note: Compared with the microsurgical clipping group;
P<0.001.
GOS scores in the two groups after the operation
The patients were followed up for 6 months after discharge. During the follow-up, the arterial embolization group had more patients with GOS score of 5 points than the microsurgical clipping group (P<0.05). See Table 6.
Table 6.
GOS scores in the two groups during the 6-month follow-up after the operation (n, %)
| Group | 5 points | 4 points | 3 points | 2 points | 1 point |
|---|---|---|---|---|---|
| Microsurgical clipping group (n=30) | 11 (36.67) | 12 (40.00) | 5 (16.67) | 1 (3.33) | 1 (3.33) |
| Arterial embolization group (n=38) | 22 (57.89)* | 11 (28.94) | 4 (7.89) | 2 (5.26) | 0 (0.00) |
Note: Compared with the microsurgical clipping group;
P<0.05.
GOS: Glasgow Outcome Scale.
mRS and BI scores in the two groups after the operation
There were no intergroup differences in the mRS and BI scores at discharge (both P>0.05). During the 6-month follow-up after discharge, the mRS score decreased and the BI score increased in both groups (both P<0.001), and the magnitude of the changes were greater in the arterial embolization group than in the microsurgical clipping group (both P<0.001). See Table 7 and Figures 1, 2.
Table 7.
mRS and Bi scores in the two groups after the operation (x̅ ± sd)
| Group | mRS score | BI score |
|---|---|---|
| Microsurgical clipping group (n=30) | ||
| At admission | 4.11±0.38 | 56.73±9.34 |
| During the 6-months follow-up | 2.12±0.31*** | 82.22±8.92*** |
| Arterial embolization group (n=38) | ||
| At admission | 4.02±0.41 | 58.42±9.27 |
| During the 6-months follow-up | 1.42±0.36***,### | 89.74±8.64***,### |
Note: Compared with the same group at hospital discharge;
P<0.001.
Compared with the microsurgical clipping group;
P<0.001.
mRS: Modified Rankin Scale; BI: Barthel Index.
Figure 1.
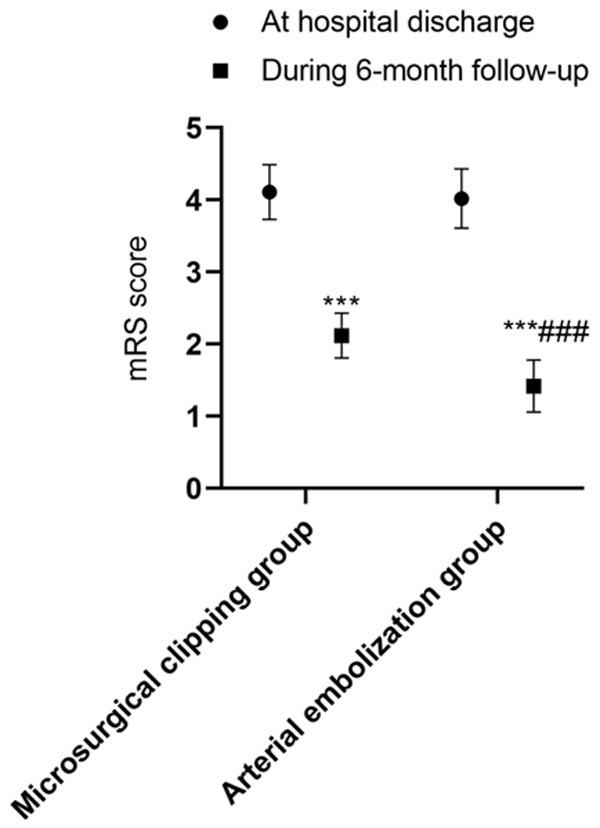
mRS scores in the two groups at discharge and during the 6-month follow-up. Compared with the same group at hospital discharge, ***P<0.001; compared with the microsurgical clipping group, ###P<0.001. mRS: Modified Rankin Scale.
Figure 2.
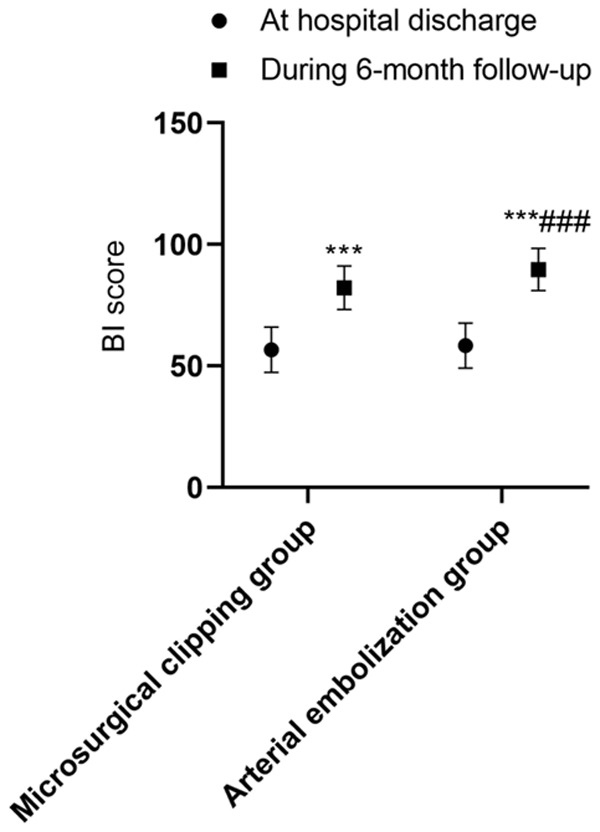
BI scores in the two groups at discharge and during the 6-month follow-up. Compared with the same group at hospital discharge, ***P<0.001; compared with the microsurgical clipping group, ###P<0.001. BI: Barthel Index.
Discussion
Microsurgical clipping of intracranial aneurysms and arterial embolization are regarded as the most effective treatment methods for ruptured anterior circulation aneurysms. However, there are still some controversies about which surgical method is more beneficial for patients. In this study, both the microsurgical clipping group and the arterial embolization group had an increased number of patients with HHC grade I and II at hospital discharge, and there was no intergroup difference in this marker, suggesting that both microsurgical clipping and arterial embolization can achieve good short-term outcome. Microsurgical clipping of intracranial aneurysms has been proven to prevent the aneurysm from re-rupture and re-bleeding, thus avoiding subarachnoid hemorrhage and helping to evacuate intracerebral hematoma [16]. Due to the development of interventional technology, arterial embolization has become a new surgical method. In this procedure, the aneurysmal cavity is embolized with a spring coil through a minimally invasive approach, and the procedure can achieve good outcomes [17,18]. Some studies have pointed out that the effective rate of arterial embolization is similar to that of microsurgical clipping of intracranial aneurysms in the treatment of anterior circulation intracranial aneurysms, and our study results are consistent with these reports [8].
At present, postoperative complications are severe issues for patients undergoing surgery for ruptured anterior circulation aneurysms. In this study, there was no intergroup difference in the total incidence of postoperative complications. However, the microsurgical clipping group had a higher incidence of intracranial infection than the arterial embolization group. This may be because the microsurgical clipping can inevitably cause surgical trauma, thus increasing the infection rate. Moreover, due to the small surgical field during the microscopic surgery, local vascular tissue can be further damaged when surgeons work on the aneurysm located deep within the brain, thereby affecting the surgical outcome and increasing the incidence of complications [19]. Some studies have reported that the incidence of bleeding of aneurysm body or parent artery during the microsurgical clipping of intracranial aneurysms and incidence of postoperative infection are closely related to the surgeon’s skill proficiency, and microsurgical clipping is not suitable for elderly patients with cardiopulmonary diseases [20]. In this study, the incidence of the vasospasm in the arterial embolization group was higher than that in the microsurgical clipping group. Some researchers have revealed that arterial embolization can cause a high recurrence rate in treating patients with large intracranial aneurysms, and these patients are likely to have cerebral vasospasm after the operation. Therefore, arterial embolization is not suitable for patients who have persistent cerebral artery spasm or high intracranial pressure [21]. The cause of vasospasm in arterial embolization may be related to the continuous stimulation of foreign matter such as the guidewire and spring coil in the arterial lumen [22]. The results of this study aligns with other studies.
Due to the prolongation of life span and the increasing demand for health services in China, the limited medical resources are becoming strained. Therefore, it is essential to find ways to both effectively treat patients and reduce their medical expenditure. In this study, although the microsurgical clipping group had longer length of hospital stay, the microsurgical clipping group had lower hospitalization cost than the arterial embolization group. These results may be due to that arterial embolization is a minimally invasive operation and has a relatively small impact on the function of arteries and corresponding nerves, which is conducive to the recovery of patients and can shorten the hospitalization. However, the medical consumables such as guidewire, micro guidewire, and spring coil used in arterial embolization are expensive, thus the hospitalization cost of this procedure is higher than that of microsurgical clipping [23].
Currently, there are still some debates on the choice of surgical methods for anterior circulation aneurysms. However, a consensus has been reached that the complete clipping rate of the microsurgical clipping is higher than the complete embolization rate of the interventional arterial embolization, and our study results are consistent with this point [24]. This may be because direct vision is provided under the microscope in the microsurgical clipping, and the advancing microscopic technology helps to increase the complete clipping rate of microsurgical clipping. According to some reports, the complete clipping rate in microsurgical clipping can reach 96% [25].
At present, most of the studies have agreed that microsurgical clipping of intracranial aneurysms and arterial embolization can reach similar short-term effects in the treatment of anterior circulation intracranial aneurysms [8]. However, the long-term effects of the two surgical methods vary between the studies. Some scholars have reported that the patients undergoing arterial embolization have lower 5-year mortality than those undergoing microsurgical clipping, but the proportion of patients at 5 years who are independent does not differ between the two groups [26]. Other studies have reported that patients undergoing microsurgical clipping can achieve the same level of recovery as those undergoing arterial embolization in the long-term [25]. In this study, the patients receiving arterial embolization achieved better scores in GOS, BI, and mRS systems during the 6-month follow-up after discharge compared with those receiving microsurgical clipping, suggesting that arterial embolization can better improve the prognosis, self-care ability, and ADL of patients with ruptured anterior circulation aneurysms than with microsurgical clipping.
There are still some limitations in the study. The sample size was small, the follow-up period was relatively short, and the effects of the two procedures on large and giant anterior circulation aneurysms were not investigated. Some scholars have pointed out that compared with microsurgical clipping, arterial embolization can achieve a higher complete occlusion rate in the treatment of large and giant anterior circulation aneurysms [27]. Therefore, more studies with a larger sample size need to be conducted in the future for verification.
In conclusion, microsurgical clipping of intracranial aneurysms and arterial embolization can achieve similar short-term effects in treating ruptured anterior circulation aneurysms. Compared with microsurgical clipping, arterial embolization can lead to shorter hospitalization, lower incidence of intracranial infection, and better patient prognosis and quality of life after the operation. However, the microsurgical clipping can bring lower hospitalization cost, higher complete occlusion rate, and lower incidence of vasospasm than arterial embolization.
Disclosure of conflict of interest
None.
References
- 1.Reaz M, Habib P, Khan S, Khan A. Risk factors and morphological differences of ruptured saccular aneurysm in different sites of anterior circulation in patients presenting with subarachnoid haemorrhage. J Nat Instit Neurosci Bangl. 2018;3:21–28. [Google Scholar]
- 2.Jin K, Chu RT, Cheng XQ. Microsurgical clip experience of anterior circulation ruptured aneurysms on 63 cases. Chin Foreign Med Res. 2019 [Google Scholar]
- 3.Cheng Z. Effect on aneurysm clipping of anterior intracranial circulation under the microscopically of pterion. J Snake. 2018 [Google Scholar]
- 4.Jia ZY, Lee SH, Kim YE, Choi JH, Hwang SM, Lee GY, Youn JH, Lee DH. Optimal guiding catheter length for endovascular coiling of intracranial aneurysms in anterior circulation in era of flourishing distal access system. Neurointervention. 2017;12:91–99. doi: 10.5469/neuroint.2017.12.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JM, Lei C, Neurosurgery DO. Short-term curative effect of endovascular embolization on patients with ruptured intracranial anterior circulation aneurysms and the influencing factors of prognosis. Hainan Med J. 2019 [Google Scholar]
- 6.Morais R, Mine B, Bruyère PJ, Naeije G, Lubicz B. Endovascular treatment of intracranial aneurysms with the p64 flow diverter stent: mid-term results in 35 patients with 41 intracranial aneurysms. Neuroradiology. 2017;59:263–269. doi: 10.1007/s00234-017-1786-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Seung WB. Delayed branching artery occlusion caused by clip rotation after intracranial aneurysm clippings. Case Rep Neurol. 2018;10:159–164. doi: 10.1159/000490375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambekar S, Khandelwal P, Bhattacharya P, Watanabe M, Yavagal DR. Treatment of unruptured intracranial aneurysms: a review. Expert Rev Neurother. 2016;16:1205–1216. doi: 10.1080/14737175.2016.1199958. [DOI] [PubMed] [Google Scholar]
- 9.Molyneux A, Kerr R International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. Stratton I, Sandercock P, Clarke M, Shrimpton J, Holman R. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. 2002;11:304–314. doi: 10.1053/jscd.2002.130390. [DOI] [PubMed] [Google Scholar]
- 10.Balik V, Yamada Y, Talari S, Kei Y, Sano H, Sulla I, Suyama D, Kawase T, Takagi K, Takizawa K, Kato Y. State-of-art surgical treatment of dissecting anterior circulation intracranial aneurysms. J Neurol Surg A Cent Eur Neurosurg. 2017;78:67–77. doi: 10.1055/s-0036-1588064. [DOI] [PubMed] [Google Scholar]
- 11.Silva MA, Lai PMR, Du R, Aziz-Sultan MA, Patel NJ. The ruptured arteriovenous malformation grading scale (RAGS): an extension of the hunt and hess scale to predict clinical outcome for patients with ruptured brain arteriovenous malformations. Neurosurgery. 2020;87:193–199. doi: 10.1093/neuros/nyz404. [DOI] [PubMed] [Google Scholar]
- 12.Ward Fuller G, Hernandez M, Pallot D, Lecky F, Stevenson M, Gabbe B. Health state preference weights for the glasgow outcome scale following traumatic brain injury: a systematic review and mapping study. Value Health. 2017;20:141–151. doi: 10.1016/j.jval.2016.09.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burn JP. Modified rankin scale. New York: Springer; 2011. [Google Scholar]
- 14.Liu F, Tsang RC, Zhou J, Zhou M, Zha F, Long J, Wang Y. Relationship of barthel index and its short form with the modified rankin scale in acute stroke patients. J Stroke Cerebrovasc Dis. 2020;29:105033. doi: 10.1016/j.jstrokecerebrovasdis.2020.105033. [DOI] [PubMed] [Google Scholar]
- 15.Escobar-de la Garma VH, Zenteno M, Padilla-Vázquez F, San-Juan D, Cerón-Morales A. Comparative analysis of aneurysm volume by different methods based on angiography and computed tomography angiography. Neurosurg Rev. 2018;41:1013–1019. doi: 10.1007/s10143-018-0943-3. [DOI] [PubMed] [Google Scholar]
- 16.Acharya A, Sudhakar P, Grewal S, Sobti S. Outcome-assessment-in-patients-with-anterior-circulation-intracranial-aneurysms-treated-with-microsurgical-clipping-a-follow-up-study July 2020 1594805294 7802740. Neurosurgery. 2020:9. [Google Scholar]
- 17.Ohshima T, Dash C, Belayev A, Yamamoto T, Goto S, Kato Y. 8-F balloon guide catheter for embolization of anterior circulation aneurysms: an institutional experience in 152 patients. Nagoya J Med Sci. 2017;79:435–441. doi: 10.18999/nagjms.79.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goertz L, Dorn F, Kraus B, Borggrefe J, Schlamann M, Forbrig R, Turowski B, Kabbasch C. Safety and efficacy of the derivo embolization device for the treatment of ruptured intracranial aneurysms. J Neurointerv Surg. 2019;11:290–295. doi: 10.1136/neurintsurg-2018-014166. [DOI] [PubMed] [Google Scholar]
- 19.Esenkaya A, Duzgun F, Cinar C, Bozkaya H, Eraslan C, Ozgiray E, Oran I. Endovascular treatment of intracranial infectious aneurysms. Neuroradiology. 2016;58:277–284. doi: 10.1007/s00234-015-1633-2. [DOI] [PubMed] [Google Scholar]
- 20.Oishi H, Arai H. The use and limitations of flow diverters in the treatment of large and giant intracranial aneurysms. Japan J Neurosurg. 2018;27:201–207. [Google Scholar]
- 21.Pumar JM, Banguero A, Cuellar H, Guimaraens L, Masso J, Miralbes S, Blanco-Ulla M, Vazquez-Herrero F, Souto M, Gelabert-Gonzalez M. Treatment of intracranial aneurysms with the silk embolization device in a multicenter study. A retrospective data analysis. Neurosurgery. 2017;81:595–601. doi: 10.1093/neuros/nyw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Durán S, Mielke D, Rohde V, Malinova V. The application of the unruptured intracranial aneurysm treatment score: a retrospective, single-center study. Neurosurg Rev. 2018;41:1021–1028. doi: 10.1007/s10143-018-0944-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Lin H, Summers R, Yang M, Cousins BG, Tsui J. Current treatment strategies for intracranial aneurysms: an overview. Angiology. 2018;69:17–30. doi: 10.1177/0003319717700503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton J, Debrun GM, Aletich VA, Bashir Q, Charbel FT, Ausman J. Follow-up angiography of intracranial aneurysms treated with endovascular placement of guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Lanzino G, Fraser K, Kanaan Y, Wagenbach A. Treatment of ruptured intracranial aneurysms since the International Subarachnoid Aneurysm Trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006;104:344–349. doi: 10.3171/jns.2006.104.3.344. [DOI] [PubMed] [Google Scholar]
- 26.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JH, Lee KS, Kim BS, Shin YS. Treatment outcomes of large and giant intracranial aneurysms according to various treatment modalities. Acta Neurochir (Wien) 2020;162:2745–2752. doi: 10.1007/s00701-020-04540-1. [DOI] [PubMed] [Google Scholar]


